PowerPoint Presentation -0.24 - The Leitzel Center
advertisement
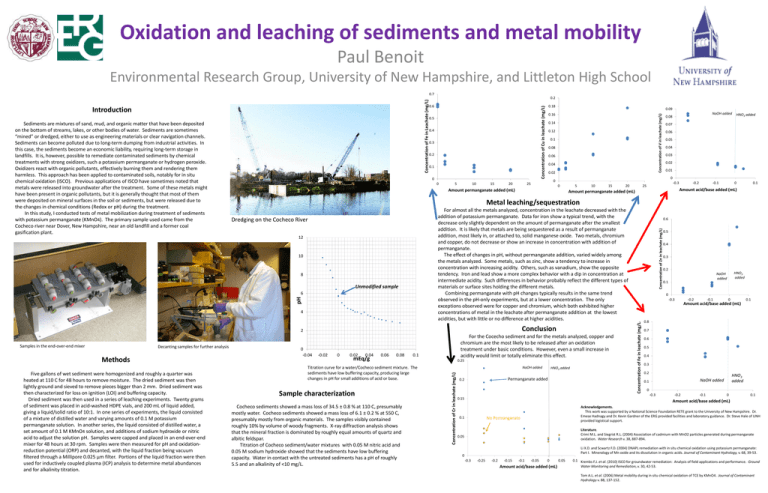
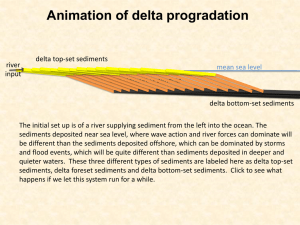
Oxidation and leaching of sediments and metal mobility Paul Benoit Environmental Research Group, University of New Hampshire, and Littleton High School 0.5 0.4 0.3 0.2 0.1 0 0 5 10 15 20 0.12 0.1 0.08 0.06 0.04 0.02 0.08 HNO3 added 0.07 0.06 0.05 0.04 0.03 0.02 0.01 0 25 NaOH added 0 0 5 10 15 20 -0.3 25 -0.2 -0.1 0 0.1 Amount acid/base added (mL) Amount permanganate added (mL) 12 pH 4 Conclusion 2 -0.02 0 0.02 0.04 mEq/g 0.06 0.08 0.1 Titration curve for a water/Cocheco sediment mixture. The sediments have low buffering capacity, producing large changes in pH for small additions of acid or base. Sample characterization Cocheco sediments showed a mass loss of 34.5 ± 0.8 % at 110 C, presumably mostly water. Cocheco sediments showed a mass loss of 6.1 ± 0.2 % at 550 C, presumably mostly from organic materials. The samples visibly contained roughly 10% by volume of woody fragments. X-ray diffraction analysis shows that the mineral fraction is dominated by roughly equal amounts of quartz and albitic feldspar. Titration of Cocheco sediment/water mixtures with 0.05 M nitric acid and 0.05 M sodium hydroxide showed that the sediments have low buffering capacity. Water in contact with the untreated sediments has a pH of roughly 5.5 and an alkalinity of <10 mg/L. 0.25 NaOH added Concentration of Cr in leachate (mg/L) 0 -0.04 For the Cocecho sediment and for the metals analyzed, copper and chromium are the most likely to be released after an oxidation treatment under basic conditions. However, even a small increase in acidity would limit or totally eliminate this effect. HNO3 added Permanganate added 0.2 0.15 0.6 Concentration of Zn in leachate (mg/L) Dredging on the Cocheco River 6 Five gallons of wet sediment were homogenized and roughly a quarter was heated at 110 C for 48 hours to remove moisture. The dried sediment was then lightly ground and sieved to remove pieces bigger than 2 mm. Dried sediment was then characterized for loss on ignition (LOI) and buffering capacity. Dried sediment was then used in a series of leaching experiments. Twenty grams of sediment was placed in acid-washed HDPE vials, and 200 mL of liquid added, giving a liquid/solid ratio of 10:1. In one series of experiments, the liquid consisted of a mixture of distilled water and varying amounts of 0.1 M potassium permanganate solution. In another series, the liquid consisted of distilled water, a set amount of 0.1 M KMnO4 solution, and additions of sodium hydroxide or nitric acid to adjust the solution pH. Samples were capped and placed in an end-over-end mixer for 48 hours at 30 rpm. Samples were then measured for pH and oxidationreduction potential (ORP) and decanted, with the liquid fraction being vacuum filtered through a Millipore 0.025 µm filter. Portions of the liquid fraction were then used for inductively coupled plasma (ICP) analysis to determine metal abundances and for alkalinity titration. 0.14 For almost all the metals analyzed, concentration in the leachate decreased with the addition of potassium permanganate. Data for iron show a typical trend, with the decrease only slightly dependent on the amount of permanganate after the smallest addition. It is likely that metals are being sequestered as a result of permanganate addition, most likely in, or attached to, solid manganese oxide. Two metals, chromium and copper, do not decrease or show an increase in concentration with addition of permanganate. The effect of changes in pH, without permanganate addition, varied widely among the metals analyzed. Some metals, such as zinc, show a tendency to increase in concentration with increasing acidity. Others, such as vanadium, show the opposite tendency. Iron and lead show a more complex behavior with a dip in concentration at intermediate acidity. Such differences in behavior probably reflect the different types of materials or surface sites holding the different metals. Combining permanganate with pH changes typically results in the same trend observed in the pH-only experiments, but at a lower concentration. The only exceptions observed were for copper and chromium, which both exhibited higher concentrations of metal in the leachate after permanganate addition at the lowest acidities, but with little or no difference at higher acidities. Unmodified sample Methods 0.16 Metal leaching/sequestration 8 Decanting samples for further analysis 0.09 Amount permanganate added (mL) 10 Samples in the end-over-end mixer 0.18 0.5 0.4 0.3 0.2 HNO3 added NaOH added 0.1 0 -0.3 Concentration of Fe in leachate (mg/L) Sediments are mixtures of sand, mud, and organic matter that have been deposited on the bottom of streams, lakes, or other bodies of water. Sediments are sometimes “mined” or dredged, either to use as engineering materials or clear navigation channels. Sediments can become polluted due to long-term dumping from industrial activities. In this case, the sediments become an economic liability, requiring long-term storage in landfills. It is, however, possible to remediate contaminated sediments by chemical treatments with strong oxidizers, such a potassium permanganate or hydrogen peroxide. Oxidizers react with organic pollutants, effectively burning them and rendering them harmless. This approach has been applied to contaminated soils, notably for in situ chemical oxidation (ISCO). Previous applications of ISCO have sometimes noted that metals were released into groundwater after the treatment. Some of these metals might have been present in organic pollutants, but it is generally thought that most of them were deposited on mineral surfaces in the soil or sediments, but were released due to the changes in chemical conditions (Redox or pH) during the treatment. In this study, I conducted tests of metal mobilization during treatment of sediments with potassium permanganate (KMnO4). The primary sample used came from the Cocheco river near Dover, New Hampshire, near an old landfill and a former coal gasification plant. 0.6 Concentration of V in leachate (mg/L) Introduction 0.2 Concentration of Cu in leachate (mg/L) Concentration of Fe in Leachate (mg/L) 0.7 -0.2 -0.1 0 Amount acid/base added (mL) 0.1 0.8 0.7 0.6 0.5 0.4 0.3 0.2 HNO3 added NaOH added 0.1 0 -0.3 -0.2 -0.1 0 0.1 Amount acid/base added (mL) 0.1 Acknowledgements. This work was supported by a National Science Foundation RETE grant to the University of New Hampshire. Dr. Emese Hadnagy and Dr. Kevin Gardner of the ERG provided facilities and laboratory guidance. Dr. Steve Hale of UNH provided logistical support. No Permanganate Literature. Crimi M.L. and Siegrist R.L. (2004) Association of cadmium with MnO2 particles generated during permanganate oxidation. Water Research v. 38, 887-894. 0.05 Li X.D. and Scwartz F.D. (2004) DNAPL remediation with in situ chemical oxidation using potassium permanganate: Part I. Mineralogy of Mn oxide and its dissolution in organic acids. Journal of Contaminant Hydrology, v. 68, 39-53. 0 -0.3 -0.25 -0.2 -0.15 -0.1 -0.05 0 Amount acid/base added (mL) 0.05 0.1 Krembs F.J. et al. (2010) ISCO for groundwater remediation: Analysis of field applications and performance. Ground Water Monitoring and Remediation, v. 30, 42-53. Tom A.L. et al. (2006) Metal mobility during in situ chemical oxidation of TCE by KMnO4. Journal of Contaminant Hydrology v. 88, 137-152.