Lecture 20
advertisement

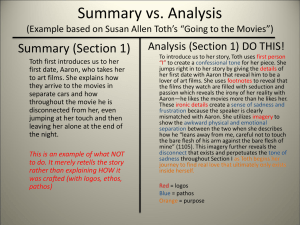
Acid-Base Reactions and Proton Accounting Lecture 20 Water • Water is virtually omnipresent at the surface of the Earth. • Consequently, there is continual reaction between water and materials at the surface (rocks, soil, atmosphere, life). • As a consequence of these reactions, water is never pure (though often pure enough that we will find it convenient to assume its concentration is 1M). • We’ll now apply our tools of physical chemistry to the the problem of aqueous solutions and their interaction with the atmosphere and, particularly, the solid Earth. Aquatic Reactions • Acid-Base H2CO3 ⇋ H+ + HCO3– • Complexation Hg2+ + H2O ⇋ Hg(OH)- + H+ • Dissolution/Precipitation KAlSI3O8 + H+ + 7H2O ⇋ Al(OH)3 + K+ + 3H4SiO4 • Adsorption/Desorption ≡S + Mn2+ ⇋ ≡S–Mn • We’ll consider each of these in turn. Importance of Acid-Base Reactions • The hydrogen and hydroxide ions are often participants in all the foregoing reactions. • As a result, these reactions are pH-dependent. o In order to characterize the state of an aqueous solution, that is, to determine how much CaCO3 a solution will dissolve, the complexation state of metal ions, or the redox state of Mn, the first step is usually to determine pH. • On a larger scale, weathering of rock and precipitation of sediments depend critically on pH. • Thus pH is sometimes called the master variable in aquatic systems. • The concentration of OH– is also known from pH since [OH–][H+] = 10-14 o at 25˚C. (Strictly speaking, it is the product of activities equal to 10-14. For simplicity, we will often assume ideality. Defining Acids and Bases • Arrhenius defined an acid as a substance that upon solution in water releases free protons. He defined a base is a substance that releases hydroxide ions in solution. • Chemists generally prefer the definition of Brønstead, who defined acid and base as proton donors and proton acceptors respectively. • The strength of an acid or base is measured by its tendency to donate or accept protons. The dissociation constant for an acid or base is a quantitative measure of its strength. For example, dissociation of HCl: HCl ⇋ H+ + ClK Diss = aH + aCl aHCl = 10 3 • Thus is a strong acid because only about 3% remains undissociated. • In contrast, for H2S ⇋ H+ + HS– • Kdiss = 10-7; very few hydrogens generally dissociate (except in very allkaline solution). Amphoteric Behavior • Metal hydroxides can either donate or accept protons, depending upon pH. For example, we can represent this in the case of aluminum as: Al(OH)2+ + H+ ⇋ Al(OH)2+ +H2O Al(OH)2+ + OH– ⇋ Al(OH)3 • Metals dissolved in water are always surrounded by solvation shells. The positive charges of the hydrogens in the surrounding water molecules are to some extent repelled by the positive charge of the metal ion. For this reason, water molecules in the solvation shell are more likely to dissociate and give up a proton more readily than other water molecules. Thus the concentration of such species will affect pH. Proton Accounting • Knowing the pH of an aqueous system is the key to understanding it and predicting its behavior. This requires a system of accounting for the H+ and OH– in the system. There are several approaches to doing this. o proton balance equation o TOTH proton mole balance equation Proton Balance Equation • The concentration of all species whose genesis caused the production of OH– are written on one side, and the concentration of all species whose genesis caused the production of H+ are written on the other side. • For water: [H+] = [OH–] • For HNO3 = H+ + NO3[H+] = [OH–] + [NO3-] Proton Mole Balance Equation • In the Morel & Hering system, H+ and H2O are always chosen as components of the system but OH– is not. The species OH– is the algebraic sum of H2O less H+ • OH– = H2O – H+ • When an acid, such as HCl, is present we choose the conjugate anion as the component, so that the acid HCl is formed from components: • HCl = Cl- + H+ • For bases, such as NaOH, we choose the conjugate cation as a component. The base, NaOH, is formed from components as follows: • NaOH = Na+ + H2O - H+ • Because we are generally dealing with dilute solutions, we assume XH2O = 1 or 55.4M (this is only 2-3% different in seawater), H2O is an implicit component; presence assumed by not written. TOTH • TOTH is the total amount of component H+, rather than the total of the species H+. • o Every species containing H+ contributes positively to TOTH while every species formed by subtracting H+ contributes negatively to TOTH. For pure water: TOTH = [H+] - [OH–] o Of course in pure water [H+] = [OH–] so TOTH = 0. • Now we dissolve CaCO3 to our solution and chose Ca2+ and CO32- as components. • o In near neutral pH, almost all the CO32- will react to form HCO3–: CO3+ + H2O = HCO3- + OH– o some Ca2+ (though generally not much) will form Ca(OH)+, so our mole balance equation will be TOTH = [H+] - [OH–] + [HCO3–] - [Ca(OH)+] Since we have not added [H+], TOTH remains 0. TOTH • Now we dissolve CO2 in our solution: • H2O + CO2 = H2CO3 o In near neutral pH, almost all the H2CO3 will react to form HCO3–: H2CO3 ⇋ HCO3- + H+ o If we chose CO2 as our component, HCO3– = CO2 + H2O - H+ TOTH = [H+] - [OH–] - [HCO3–] • This time HCO3- contributes negatively. • • Every species containing H+ contributes positively to TOTH while every species formed by subtracting H+ contributes negatively to TOTH. How we write the TOTH equation depends on how we defined components. • Since we have not added [H+], TOTH remains 0.





![pH = - log [H + ]](http://s2.studylib.net/store/data/005622524_1-002df1ea50d2a849b15deb604928664e-300x300.png)

