Aqueous Complexes (or speciation)
advertisement
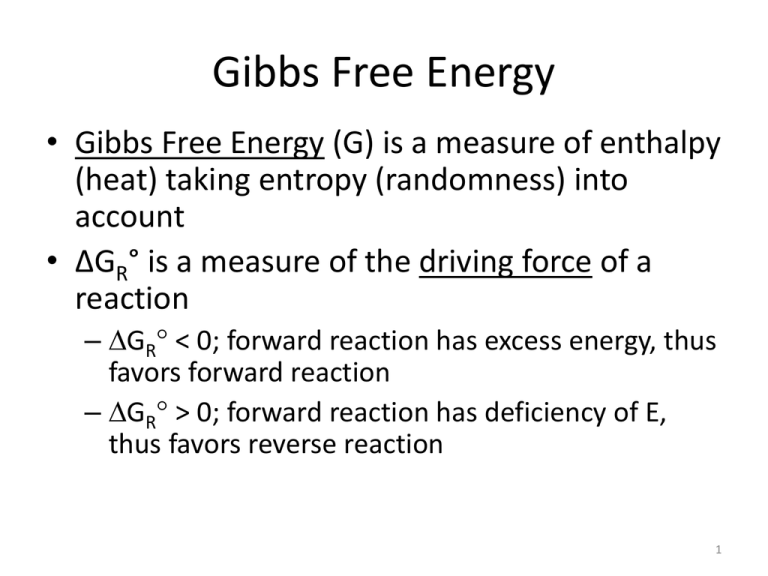
Gibbs Free Energy • Gibbs Free Energy (G) is a measure of enthalpy (heat) taking entropy (randomness) into account • ΔGR° is a measure of the driving force of a reaction – GR < 0; forward reaction has excess energy, thus favors forward reaction – GR > 0; forward reaction has deficiency of E, thus favors reverse reaction 1 Gibbs Free Energy • Two ways to calculate – GR = ΔHR - T ΔSR – GR = niGfi(products) – niGfi(reactants) • GR and Keq are related: – GR = -RT ln Keq – GR = -5.708 log Keq at 25°C 2 Activity • To apply equilibrium principles to ions and molecules, we need to replace concentrations with activities to account for ionic interactions – ai = i Ci – a < C in most situations 3 Activity • Activity coefficients () are a function of ionic strength – I = ½ mi zi2 • Use Debye-Hückel or extended Debye-Hückel equation (unless saline solution) – log = -Az2I½ – log = - Az2I½ (1 - aBI½) 4 Aqueous Complexes • Complex: chemical association of 2 or more dissolved species to form a 3rd dissolved species • Some examples: – – – – Al3+ + OH- Al(OH)2+ Al(OH)2+ + OH- Al(OH)2+ Ca2+ + SO42- CaSO4(aq) Ca2+ + HCO3- CaHCO3+(aq) • Note: ΣCa in solution = Ca2+ (ion) + CaSO4° + CaHCO3+ + any other Ca-containing complexes • Aqueous complex distinguished from solid of same composition by subscript (aq) or superscript ° 5 Aqueous Complexes • 2 main types – Ion pairs – Coordination compounds 6 Ion Pairs • Cations and anions form associations in solution because of electrostatic attraction – Associations are weak bonds; form and decompose rapidly in response to changes in solution chemistry – Generally the greater the concentration, the greater the amount of ion pairs 7 Coordination Compounds • Ions surrounded by sphere of hydration; one or more water molecules displaced by ligands – Ligand = ion (usually anion) or molecule that binds to a central atom (usually a metal) – If a ligand can bind at more than one site, it is called a chelate, and the bond is stronger – Some coordination-type complexes are very stable • e.g., used in cleaning metal waste (EDTA, NTA) 8 Importance of complexes • Increase mineral solubility by decreasing effective concentrations – Equilibrium calculations are usually made with uncomplexed species – Analytical instruments usually measure total amount (uncomplexed + complexed) of a species • ΣmCa = mCa2+ + mCaHCO3+ + mCaSO4° + … – ΣmCa = measured amount – mCa2+ used in thermodynamic calculations – We can’t directly measure mCaHCO3+, mCaSO4°: must calculate 9 Importance of complexes • Many elements exist dominantly as a complex or complexes) – Usually those with low solubilities such as metals • As, Fe, Al, Pb, Hg, Cu, U to name a few • e.g., As5+ usually exists as an oxyanion (H2AsO4-) and As3+ usually exists as an uncharged species (H3AsO3°) 10 Importance of complexes • Adsorption can be increased or inhibited – Adsorption is usually a weak attraction of charged species to aquifer solids – Charged complexes more likely than uncharged complexes to adsorb and be removed from solution • Bioavailability and toxicity – Some complexes of essential nutrients may pass straight through living organisms – Some complexes of toxic species may pass straight through living organisms • CH3Hg+ most toxic form; elemental Hg much less toxic 11 Complexes: General Observations • Low solubility elements exist predominantly as complexes (e.g., metals) • Complexation tends to increase with increasing I – More potential ions to complex with – Species are closer together • Mineral solubility also increases with increasing I – Combined effects of complexation and activity; ions in solution less reactive • Increasing charge density (charge per surface area) results in stronger complexes – Charge density increases with increasing valence or decreasing atomic radius – Function of valence and ion size 12 Complexes and Thermodynamics • The presence and stability of complexes can be predicted using thermodynamics – Ca2+ + SO42- CaSO4(aq) – Kassoc = association constant (also Ka); also called stability constant – The larger the Kassoc , the more stable the complex • Kassoc have smaller ranges compared to Keq, probably because bonds are weak • As with Keq, Kassoc values have been determined in the lab and are included in thermodynamic databases of geochemical models 13 Calculating complexes • Need complete chemical analyses to calculate concentrations of complexes – If important complexing species are missing, data interpretation may be in error – Speciation often a function of pH, so must have accurate field pH 14 Complexation Example • How much is Ca2+ decreased by complexation with SO42-? 15 pH • pH = -log[H+] • Many reactions involve H+ – Silicate and carbonate weathering – Sulfide weathering (acid mine drainage) – Dissociation of water molecule – Adsorption – Microbial processes; e.g., denitrification – Amphoteric oxyhydroxides; Fe(OH)3, Al(OH)3 – Aqueous complexes 16 pH as Master Variable • pH is key parameter affecting species distribution • Therefore useful to consider activity of other species with respect to pH • pH is a “master variable” 17 Acids and Bases 18 Definitions • Acid is a compound that releases H+ when dissolved in water (proton donor) • Base is a compound that releases OH- when dissolved in water (proton acceptor) – Acids and bases can be liquids or solids • e.g., H2CO3 HCO3- + H+ – H2CO3 donates a proton when it dissociates = Acid • HCO3- + H+ H2CO3 – HCO3- accepts a proton = Base 19 Definitions • Some species can act as both acid and base, depending on reaction • HCO3- + H+ H2CO3 – HCO3- accepts a proton = Base • HCO3- CO32- + H+ – HCO3- donates a proton = Acid 20 Acid/Base Strength • Strength is a measure of the tendency of an acid or base to give or accept protons • Strong acids/bases release all/most available H+/OH– Strong acids almost completely dissociate in water; large Ka • • • • HCl H+ + ClSulfuric acid: H2SO4 – acid rain (burning fossil fuels), AMD Nitric acid: HNO3 – acid rain, nitrification (NH4+ NO3-) Not usually large natural source of acid – Strong bases: hydroxides of alkali metals (Li, Na, K, Rb, Cs, Fr) and many alkaline earths (Mg, Ca, Sr, Ba, Ra) 21 Alkali Metals Alkaline Earths 22 Acid/Base Strength • Weak acids/bases release only a small fraction of available H+/OH– Acetic acid (CH3COOH), H2CO3, H3PO4, H4SiO4 • Small Ka – Ammonium hydroxide (NH4OH), nickel hydroxide (Ni(OH)2) • In real world geochemistry, we’re mainly interested in weak acids and bases • Strength has nothing to do with concentration – HCl is still a strong acid even if it is greatly diluted – Acetic acid is still weak even if in a concentrated solution • Usually measure using Normality (N) 23 Important Acids in Groundwater • Carbonic acid: H2CO3 – CO2 – Dominant source of H+ in most groundwater • Silicic acid: H4SiO4 – mineral weathering • Acetic acid: CH3COOH – natural and anthropogenic (landfills); organic acid • Other organic acids (formic, oxalic) • Phosphoric: H3PO4 24 Dissociation of Silicic Acid • H4SiO4 ↔ H+ + H3SiO4– 1st dissociation : – Ka1 is small – At pH 7: • H3SiO4- ↔ H+ + H2SiO42– 2nd dissociation: – Ka2 is very small 25 Dissociation of Silicic Acid (cont.) • H2SiO42- ↔ H+ + HSiO43– 3rd dissociation: – miniscule • HSiO43- ↔ H+ + SiO44– 4th dissociation: < miniscule 26 Dissociation Reactions • Dissociation reactions reach equilibrium very quickly – e.g. CH3COOH CH3COO- + H+ – • Ka = 1.76 x 10-5 at 25°C, 1 atm • Very small number, most remains undissociated – Knowing Ka and the initial concentration of CH3COOH, we can calculate how much dissociates 27 Example • Assume 0.1 moles of acetic acid is dissolved in 1 L H2O, determine fraction (x) that dissociates… – Assume γ = 1 28 Dissociation of Water • H2O H+ + OH- (or H2O + H+ ↔ H3O+) – Kw = [H+] [OH-] = 1 x 10-14 at 25°C • Remember that [H2O] = 1 • Small dissociation constant, but nearly unlimited source of H+ or OH- – For pure H2O at 25°C, [H+] = [OH-] = 10-7 mol/L 29 Dissociation of Water • H2O H+ + OH– pH = -log [H+] • • • • • Useful for reflecting on progress of chemical reactions Easy to measure pH = 7 for pure water at 25°C, 1 atm Usually we consider pH values between 0 and 14 pH for most natural waters is between 6 and 9 – We can also define pOH = -log [OH-] • Not widely used • At 25°C, pOH = 14 - pH 30 pH in the Environment • Weak acids/bases do not control the pH of the natural environment, but respond to it • pH is an environmental variable determined by all of the simultaneous equilibria existing in a given environment 31 Calculating pH of Acids and Bases • What is the pH of 0.1 M acetic acid? – CH3COOH CH3COO- + H+ – Recall we calculated that [H+] = 1.32 x 10-3 mol/L – pH = 2.88 – Note that even though acetic acid is a weak acid, the pH of a fairly concentrated solution of it is quite low 32 Polyprotic Acids/Bases • A weak acid or base that can yield 2 or more H+ or OH- per molecule of acid/base is polyprotic – H2S(aq) H+ + HS• – HS- H+ + S2• – Note that 1st reaction contributes much more H+ (K1 >> K2) – Other examples: H2CO3, H2SO4, H3PO4 33 Determining concentrations of species for polyprotic acids/bases • Dissolve 0.1 moles of H2S in pure water at 25°C; what are the concentrations of all the aqueous species? – Again, we’ll assume γ = 1 34 Sparingly Soluble Bases • Many bases do not readily dissolve in water • e.g., Brucite (Mg(OH)2) solubility • Mg(OH)2(s) Mg(OH)+ + OH-: Keq = 10-8.6 – Let’s calculate the concentrations of species as a function of pH 35 Brucite solubility • Plot activity of Mg species vs. pH to get an Activity Diagram – log [Mg(OH)+] = 5.4 – pH – log [Mg2+] = 16.8 – 2pH – – get straight lines intersecting at pH = 11.4 • pH = 14 – 2.6 = 11.4 – Lines represent equilibrium between the two species/compounds on opposite sides – Mg2+ dominates at pH < 11.4 (most geologic environments) 36 0 Dissociation and pH –2 –4 ++ Mg –6 Diagram Mg , T = 25 °C , P = 1.013 bars, a [H2 O] = 1 –8 + MgOH –10 ++ log a Mg ++ Brucite 25°C 0 2 4 6 8 pH 10 12 14 Walt Mon Feb 06 2006 37 Dissociation and pH • Dissociation of weak acids/bases controlled by pH • Rewrite mass action equations for H2S – H2S(aq) H+ + HS- 38 Dissociation and pH – Can do same for HS- vs. S2– HS- H+ + S2– Such relationships occur for all weak acids and bases – Knowing the total amount of S and pH, we can calculate activities of all species and generate curves 39 Total S = 10-4 M –2 -- Some species w/ SO4 (log activity) –4 H2 S(aq) HS - S -- –6 pH = 12.9 pH = 7 –8 –10 –12 –14 –16 –18 –20 2 3 4 5 6 7 8 9 10 11 12 13 14 pH Walt Tue Feb 14 2006 40 Total DIC = 10-1 M 0 - CO2 (aq) -- HCO3 CO3 pH = 10.33 pH = 6.35 –4 –6 - Species with HCO3 (log molal) –2 –8 –10 –12 –14 –16 2 3 4 5 6 7 8 9 10 11 12 pH Walt Tue Feb 21 2006 41








