Drawing Organic Structures Functional Groups
advertisement

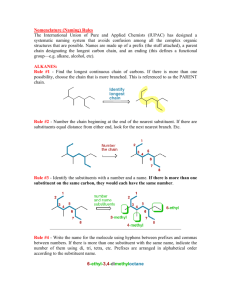
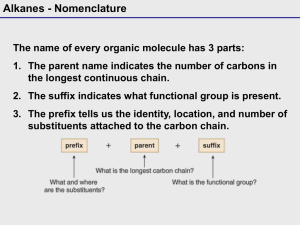
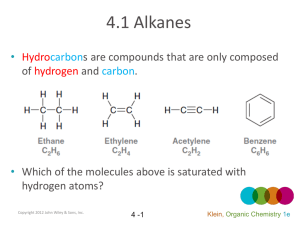
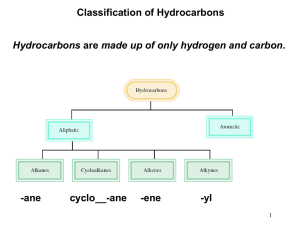
NOMENCLATURE Dr. Clower CHEM 2411 Spring 2014 McMurry (8th ed.) sections 3.2, 3.3, 3.4, 4.1, 4.2, 7.3, 7.4, 9.1, 10.1, 15.1, 17.1,18.1, 18.8, 19.1, 20.1, 21.1, 24.1, Appendix A Nomenclature • The naming of organic molecules • IUPAC system • All names have Substituents-MainChain • Substituents are groups attached to the main chain • MainChain consists of Parent-Infix-Suffix • Parent tells you number of carbons in main chain • Infix tells you if the carbon-carbon bonds are single bonds (saturated molecule) or double or triple bonds (unsaturated molecules) • Suffix tells you the functional group of your molecules Parent Number of carbons Parent name 1 meth- 2 eth- 3 prop- 4 but- 5 pent- 6 hex- 7 hept- 8 oct- 9 non- 10 dec- 11 undec- 12 dodec- Straight-chain alkanes Infix • “-an-” = only carbon-carbon single bonds (saturated) • “-en-” = an alkene (double bond) is present (unsaturated) • “-yn-” = an alkyne (triple bond) is present (unsaturated) Suffix Functional group Suffix alcohol -ol ether ether amine -amine nitrile -nitrile thiol -thiol sulfide sulfide aldehyde -al ketone -one carboxylic acid -oic acid ester -oate amide -amide acid chloride -oyl chloride acid anhydride -oic anhydride Nomenclature • Consider the molecule 2-hexanol. • How many carbons in the main chain? • Saturated or unsaturated? • What functional group? • Consider the molecule octanoic acid. • How many carbons in the main chain? • Saturated or unsaturated? • What functional group? • Consider the molecule 4-methyl-2-pentanamine. • How many carbons in the main chain? • Saturated or unsaturated? • What functional group? • What is 4-methyl? Substituents • Groups off of main chain (branches) • Parts of larger compounds (not stable by themselves) • Alkyl groups = Alkane – H • “-ane” changes to “-yl” • Methyl • methane – H • CH4 – H • CH3─ • Ethyl • ethane – H • CH3CH3 – H • CH3CH2─ Substituents • Propane – H gives two possible alkyl groups • Propyl • Isopropyl • Similarly, there are multiple alkyl groups with 4 carbon atoms, 5 carbon atoms, etc. Alkyl groups you need to know • Methyl • Pentyl (amyl) • Ethyl • Isopentyl (isoamyl) • Propyl • Neopentyl • Isopropyl • tert-pentyl • Butyl • Hexyl, heptyl, etc. • Isobutyl • Halogens • Fluoro • Chloro • Bromo • Iodo • sec-butyl • tert-butyl (t-butyl) Naming alkanes 1. Find the parent chain • Longest continuous chain of C atoms • Name of chain = parent name • If 2 chains of equal length, parent is the chain with more substituents 2. Number each carbon in the parent chain • Start from the end closest to the first substituent • If there are substituents equal distance from both ends, number from the end nearest the second substituent Naming alkanes, cont. 3. Name and number substituents • Name = alkyl group name • Number = point of attachment to parent chain • Two substituents on the same C get the same number 4. Write the name as a single word • Substituents before parent name (include #) • Separate # and word with hyphen • Separate two numbers with a comma • List substituents in alphabetical order • Multiple identical substituents use prefixes di, tri, tetra, penta, hexa • DO NOT use sec, tert, di, tri, etc. when alphabetizing • DO use iso, neo when alphabetizing Examples Structure Name Examples • Draw 3-ethylpentane. • Draw all the constitutional isomers with the molecular formula C6H14. Name each isomer. Examples Structure Name Examples Structure Name Naming Cycloalkanes • Unsubstituted cycloalkanes: • Add “cyclo” to the parent name • Bicycloalkanes • Two rings • Most common is norbornane • Bicyclo[2.2.1]heptane Naming Cycloalkanes • Substituted cycloalkanes: • Parent = ring or substituent (whichever has more carbons) • Number the substituents • Do not need to show number if only one substituent on a ring • If two substituents, start with the first alphabetically, number in the direction of the second substituent • If more than two substituents, number so that the substituents have the lowest set of numbers Examples Structure Name Examples Structure Name Cycloalkane Stereoisomers • Cycloalkanes are roughly planar • Substituents are either above or below this plane • Shown with dash and wedges • Dash = back; wedge = forward • In a disubstituted cycloalkane, two substituents on the same side (both back or both forward) are cis. Two substituents on opposite sides (one back, one forward) are trans. • Cis and trans versions of the same molecule are stereoisomers Examples Structure Name • Do these pairs represent constitutional isomers, cis- trans stereoisomers or the same compound? (a) (b) Alkene Nomenclature • Similar to alkanes • Change infix from “-an-” to “-en-” Naming Alkenes • For larger alkenes: 1. Parent is longest C chain containing both carbons of C=C 2. Number chain so C=C has lowest possible number • If the double bond is equidistant from both ends, start numbering at end nearest the first substituent • Show location of C=C by first number • Alkenes with >1 C=C use “-adiene”, “-atriene”, etc. in place of “-ene” and show location of all double bonds 3. Name and number substituents and write the full name • Example: Examples Structure Name Cycloalkenes • The carbon atoms of the C=C are numbered 1 and 2 • Number ring in direction to give first substituent lowest possible number Structure Name Alkene substituents Substituent Name CH2═ methylene CH2═CH─ vinyl CH2═CH─CH2─ allyl Alkene Stereoisomers • Cis-trans stereoisomers • Seen with disubstituted alkenes • Cis means groups are on the same side of the double bond; trans means groups are on opposite sides • Cannot convert through bond rotation • Example: 2-butene Examples • Are the following alkenes cis, trans, or neither? (a) (b) (c) Alkyne Nomenclature • Similar to alkenes • Change infix from “-en-” to “-yn-” Naming Alkynes • For larger alkynes: 1. Parent is longest C chain containing both carbons of C≡C 2. Number chain so C≡C has lowest possible number • If the triple bond is equidistant from both ends, start numbering at end nearest the first substituent • Show location of C≡C by first number • Alkynes with >1 C≡C use “-adiyne”, “-atriyne”, etc. in place of “-yne” and show location of all double bonds 3. Name and number substituents and write the full name • Example: Enyne • Contains both alkene and alkyne • Number from end closest to first multiple bond (either C=C or C≡C) and show both numbers • If the C=C and C≡C are equidistant from the ends, C=C gets the lower number • Examples: Structure Name Naming Aromatic Compounds • Benzene • Monosubstituted benzenes • Substituent name + “benzene” Common Benzene Compounds Benzene Nomenclature • If substituent has greater than 6 carbons, it becomes the parent, and benzene is called a phenyl group • Benzene substituents: Disubstituted Benzenes • ortho (1,2) • meta (1,3) • para (1,4) Naming Disubstituted Benzenes • If one substituent is part of a common name, that name is the parent and that substituent is at carbon 1 • If neither substituent is part of a common name, list the substituents in alphabetical order (first alphabetically is at carbon 1) • If both substituents are part of common name, use this order of priority to determine the parent name: -CO2H > -CHO > -OH > -NH2 > -CH3 Examples Structure Name Examples Structure Name Naming Polysubstituted Benzenes • With 3 or more substituents do not use ortho, meta, para • Number ring to give smallest set of numbers • If a common name, use as parent (substituent at carbon 1) • List substituents in alphabetical order Examples Structure Name Another Aromatic Compound • Pyridine • If substituted, nitrogen is atom 1 of the ring. Number in direction of other substituents. Naming Alcohols • Acyclic alcohols 1. Parent chain is longest chain containing C bonded to –OH 2. Change suffix from “-e” to “-ol” 3. Number from end closest to –OH. Show location of –OH. 4. Name/number substituents • Cyclic alcohols 1. Ring is the parent 2. Number ring so –OH is at carbon 1 and other substituents have lowest possible numbers. You do not need to show the location of the –OH. 3. Name/number substituents. Naming Alcohols • Multiple hydroxyl groups • Two –OH groups is a diol; 3 is a triol • Two adjacent –OH groups is a glycol • Name as acyclic alcohols, except keep the “-e” suffix and add “-diol” • Indicate numbers for all –OH groups • Unsaturated alcohols (enol or ynol) 1. Parent chain contains carbon bonded to –OH and both carbons of C=C or C≡C 2. Suffix is “-ol”, infix is “-en-” or “-yn-” 3. Number chain so –OH has the lowest number 4. Show numbers for –OH and the unsaturation 5. Name/number substituents Examples Structure Name Examples Structure Name Naming Thiols • Thiols are sulfur analogs of alcohols • Name like alcohols, but keep the “-e” and use “-thiol” in place of “-ol” Naming Amines 1. Parent chain is longest containing C bonded to –N 2. Change suffix “-e” to “-amine” 3. Number from end closest to –N. Show location of –N. 4. Name/number substituents Examples Structure Name Naming Aldehydes • Parent chain contains carbon of CHO • Suffix is “-al” • CHO carbon is carbon 1 (do not need to show in name) Naming Aldehydes • Cyclic molecules with –CHO substituents • -CHO is bonded to carbon 1 of ring • Add “carbaldehyde” to end of ring parent name Naming Ketones • Parent chain contains carbon of carbonyl; suffix is “-one” • Number so carbonyl has lowest number • Cyclic ketones carbonyl is carbon 1 of the ring • Some more common names: Examples Structure Name Order of Precedence of Functions • Used when more than one functional group in a Increasing precedence molecule Functional Group Suffix (High Precedence) Prefix (Low Precedence) -CO2H -oic acid - -CHO -al formyl- -C(O)- -one oxo- -OH -ol hydroxy- -NH2 -amine amino- Examples Structure Name Naming Carboxylic Acids • Parent chain contains carbon of –CO2H • Suffix is “-oic acid” • –CO2H is carbon 1 Naming Carboxylic Acids • Cyclic molecules with –CO2H substituents • –CO2H is bonded to carbon 1 of ring • Add “carboxylic acid” to end of ring parent name Examples Structure Name Naming Acid Chlorides • Name corresponding carboxylic acid • Change “-ic acid” to “-yl chloride” • Examples: Structure Name Naming Acid Anhydrides • If R = R’, name carboxylic acid RCO2H. Replace “acid” with “anhydride” • If R ≠ R’, list the two acids alphabetically and add the word “anhydride” • Examples: Structure Name Naming Esters • Name alkyl group bonded to oxygen (R’) • Name carboxylic acid RCO2H • Change “-ic acid” to “-ate” • Examples: Structure Name Naming Amides • Name corresponding carboxylic acid • Change “-oic acid” to “-amide” • Examples: Structure Name Example • DMF (N,N-dimethylformamide) is a common solvent in organic chemistry. Draw the structure of DMF. Naming Nitriles • Two methods 1. Nitrile carbon is carbon 1 of parent chain. Add “-nitrile” to end of alkane name. 2. Name as carboxylic acid derivative. Replace “-ic acid” with “-onitrile” Common Name System • Can be used for some simple molecules • Alkyl halides, alcohols, amines • Name alkyl group • Add “chloride” or “bromide” or “alcohol” or “amine” • Examples: Structure CH3Cl (CH3)2CHBr CH3CH2OH CH3CH2NHCH3 (CH3CH2)2NH Name Common Name System • Ketones, ether • Name both alkyl groups bonded to carbonyl (ketone), oxygen (ether), or sulfur (sulfide) • Add “ketone” or “ether” or “sulfide” • Examples: Structure Name