What is Crystallography?
advertisement

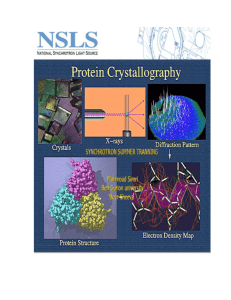
What is Crystallography? The science that examines the arrangement of atoms in solids. "crystallography" derives from the Greek words crystallon "cold drop, frozen drop" What is a crystal? crystalline if the atoms or ions that compose it are arranged in a regular way (Brief) History of Crystallography Johannes Kepler hypothesized (1611) that the hexagonal symmetry of snowflake crystals was due to a regular packing of spherical water particles. X-rays were discovered by Wilhelm Conrad Röntgen (1895) Nobel prize in physics in 1901 Max von Laue proposed that a crystal would act in a similar manner to a diffraction grating (1912) William Henry Bragg and William Lawrence Bragg (born SA!) derived a formula which describes how crystals diffract (1912). Joint Nobel prize 1915. Copper sulfate Crystal Diffraction Data Can be measured in different ways but one of the most common is as powders. Each structure gives a unique diffraction data set of peak intensities and spacings. Diamond Graphite Why is Crystallography Important? Earth Sciences – exploration, minerals processing, minerals, high pressure/temperature crystallisation… Archaeology – paints , residues, ceramics…. Materials science – solar panels, microelectronics, semi-conductors… Life sciences – DNA, proteins, drugs design… Forensic science – soils, powders, paints, explosives, poisons … Metallurgy – phases, hardening, failure, corrosion, heat treatment… Demo - Crystal Growth Supersaturation drives crystal growth. Supersaturation is a measure of how much greater the solution concentration is than at equilibrium. Can change supersaturation by changing: Concentration (add less or let solution evaporate slowly); Temperature – supersaturation increases on decreasing temperature. Blue crystals: CuSO4.5H2O Solubility 32 g/100 ml H2O at 20 °C. To make crystals, add 25 g solid into 50 ml water, heat and stir the solution. These crystals are very easy to grow and started growing within 2-3 hours after the solution cooling down. Brown crystals: Fe(NO3)3.9H2O Solubility 138 g/100 ml H2O at 20°C . To make crystals, add 70 g solid into 40 ml water, heat and stir the solution. This one took more than 1.5 day to have crystals grow on the string. Green crystals: NiCl2.6H2O Solubility 254 g/100 ml H2O at 20°C. To make crystals, add 75 g solid into 25 ml water, heat and stir the solution. Took around one day to have crystals on the string. Demo - Diffraction n = d Sinθ Wavelength 632.8 nm Diffraction angle, n=1 3.42° n=2 6.87° n=3 10.33 ‘lattice’ spacing e.g. 2400 lines/inch 10,581.6 nm Therefore can work out thickness of hair. Measure angle to regions of destructive interference. Does hair thickness vary between people? n =1 θ Diffraction Facilities Most common form of sample for phase identification and quantification. Microdiffractometer spatial resolution (0.001 mm) Single crystal system for analysis of the structure of a single phase Crystallography Now – Synchrotrons We have one in Melbourne! 216 m in diameter. (also neutrons and electrons)