REDOX Notes
advertisement

Unit 7: Redox &
{
Electrochemistry
What information
does the oxidation
number give you?
Why electrochemistry?
REDOX reactions are important in …
• Purifying metals
(e.g. Al, Na, Li)
C3H8O + CrO3 + H2SO4
Cr2(SO4)3 + C3H6O + H2O
• Producing gases
(e.g. Cl2, O2, H2)
• Electroplating metals
• Electrical production (batteries, fuel cells)
• Protecting metals from corrosion
• Balancing complex chemical equations
• Sensors and machines (e.g. pH meter)
What is Redox?
REDOX stands for REDuction/OXidation
Oxidation is often thought of as a combination of a
substance with oxygen (rusting, burning)
e-
Oxidation refers to a loss of
Reduction refers to a gain of e-
Remember:
LEO the lions
says
GERRRRRR!
Loss Electrons = Oxidation
Gain Electrons = Reduction
Reactions
What is happening to the Fe atom?
Fe is going from 0 to +3 oxidation #
It must be losing electrons
Loss of Electrons = Oxidation
In conclusion, the iron atom is being oxidized
Reactions
What is happening to the Sulfur atom?
S is going from 0 to -2 oxidation #
It must be gaining electrons
Gain of Electrons = Reduction
In conclusion, the sulfur atom is being reduced
Oxidation Numbers
- the charge an atom would have if the electrons belonged to
the more EN atom
- there are a few rules to help us out
Na
Na0
H2
H20
F2
F20
Oxidation Numbers
- the charge an atom would have if the electrons belonged to
the more EN atom
H is ALWAYS +1 (for us)
Disclaimer – there are compounds where H has a -1
oxidation number, but we don’t deal with them at this
level of chemistry.
Oxidation Numbers
- the charge an atom would have if the electrons belonged to
the more EN atom
O is ALWAYS -2 (for us)
Oxidation Numbers
- the charge an atom would have if the electrons belonged to
the more EN atom
Na+1
Calcium ion
Ca+2
Sodium ion
Sulfur ion
S-2
Nitrogen ion
N-3
Oxidation Numbers
- the charge an atom would have if the electrons belonged to
the more EN atom
NaCl
Na+1Cl-1
AsI5
As+5I5-1
Cu(NO3)2
Cu+2(N+5O3-2)2
H2Cr2O7
H2+1Cr2+6 O7-2
Oxidation Numbers
- the charge an atom would have if the electrons belonged to
the more EN atom
(SO4)-2
(S+6O4-2)-2
)-
(N+5O3-2)-
)-2
(Cr2+3O4)-2
(NO3
(Cr2O4
Oxidation Numbers
Do the five problems on your notes sheet
a. Cr2O3
d. KCl
b. H2Cr2O7
e. Mg(OH)2
c. AsCl5
What things are conserved
during a chemical reaction?
What is reduced/oxidized?
Identify in the following reactions what is
oxidized and what is reduced
2K + Cl2 2KCl
K0 – goes from 0 to +1, it is oxidized
Cl0 – goes from 0 to -1, it is reduced
Practice
Identify in the following reactions what is
oxidized and what is reduced
2NaCl + 3SO3 Cl2 + SO2 + Na2S2O7
Cl-1 – goes from -1 to 0, it is oxidized
S+6 – goes from +6 to +4, it is reduced
Practice
Identify in the following reactions what is
oxidized and what is reduced
Zn + Pb+2(aq) Zn+2(aq) + Pb
Zn0 – goes from 0 to +2, it is oxidized
Pb+2 – goes from +2 to 0, it is reduced
a. C + H2SO4 CO2 + SO2 + H2O
b. HNO3 + HI NO + I2 + H2O
c. KMnO4 + HCl MnCl2 + Cl2 + H2O +
KCl
d. Sb + HNO3 Sb2O5 + NO + H2O
e. HCl + MnO2 MnCl2 + H2O + Cl2
a. C + H2SO4 CO2 + SO2 + H2O
b. HNO3 + HI NO + I2 + H2O
c. KMnO4 + HCl MnCl2 + Cl2 + H2O + KCl
d. Sb + HNO3 Sb2O5 + NO + H2O
e. HCl + MnO2 MnCl2 + H2O + Cl2
1. C + 2Cl2 CCl4
Ox –
Red –
2. H2 + Cl2 2HCl
Ox –
Red –
3. 2P + 3Cl2 2PCl3
Ox –
Red –
4. C + H2O CO + H2
Ox –
Red –
5. Fe + 3Cl2 2FeCl2
Ox –
Red –
6. 2Al + 3Br2 2AlBr3
Ox –
Red –
7. Pb + 2HCl PbCl2 + H2
Ox –
Red –
8. SiO2 + 2C Si + 2CO
Ox –
Red –
9. CO2 + 2Mg 2MgO + C
Ox –
Red –
10. H2SO4 + Zn ZnSO4 + H2
Ox –
Red -
Identify what atom is
oxidized and what atom is
reduced:
Fe + 2HCl FeCl2 + H2
HALF REACTIONS
Write both half reactions for the following
reaction:
Cu + AgNO3 Cu(NO3)2 + Ag
Reduction: Ag+ Ag
Ag+ + 1e- Ag
Cu Cu+2
Cu Cu+2 + 2eCu - 2e- Cu+2
Oxidation:
HALF REACTIONS
Write both half reactions for the following
reaction:
HNO3 + I2 HIO3 + NO2
Reduction: N+5 N+4
N+5 + 1e- N+4
I20 I+5
I20 2I+5 + 10eI20 - 10e- 2I+5
Oxidation:
Half Reactions
Write both half reactions for the following
reaction:
Sn + AgNO3 Sn(NO3)2 + Ag
Reduction: Ag+1 Ag0
Ag+1 + 1e- Ag0
Sn0 Sn+2
Sn0 Sn+2 + 2eSn0 - 2e- Sn+2
Oxidation:
Redox Lab
2 Al + 3 CuCl2
Mass
GFM
Moles
3 Cu
+ 2 AlCl3
If you were to react Cu and
Nickel(II) Chloride what would
the products be? How much
metal could you make if you
started with 2.00g of Cu?
Oxidizing and Reducing Agents
Oxidizing Agent
- causes the oxidation of another atom
- it is actually the atom that is REDUCED
- oxidation number decreases
Reducing Agent
- causes the reduction of another atom
- it is actually the atom that is OXIDIZED
- oxidation number increases
Ca + Cl2 CaCl2
What is the O.A.? Cl
What is the R.A.? Ca
PRACTICE
In the equation below, identify what is oxidized what is
reduced. Also identify the oxidizing and reducing agent.
4HCl + MnO2 MnCl2 + 2H2O + Cl2
Oxidized:
Cl-
Reduced:
Mn+4
Oxidizing Agent:
Mn+4
Reducing Agent:
Cl-
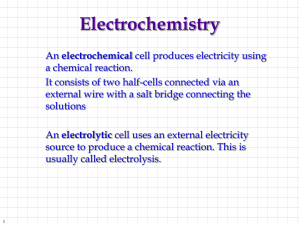
Electrochemical Reactions
Deals with chemical reactions that either produce electricity or
need electricity to occur!
There are 2 types of ELECTROCHEMICAL CELLS.
Some things that are the same for both types of cells:
1. The RED CAT GETS FAT!
Anorexic Ox
2. Electrons always flow from the anode to the cathode!
Half Reactions
2e-
2e-
2e-
2e-
2e-
2e-
2e-
2e-
2e-
2e-
2e-
Zn electrode
Cu electrode
Lose e- (Table J)
Gains e- (Table J)
Oxidized
Anode (-)
Cu+2
Zn+2
Zn
Reduced
Cathode(+)
Zn+2
ZnSO4
Cu+2
CuSO4
Will this go on forever?
Cu
Half Reactions
Zn electrode
Cu electrode
Lose e- (Table J)
Gains e- (Table J)
Oxidized
Reduced
Anode (-)
Cathode(+)
Zn
Cu
Zn+2
ZnSO4
CuSO4
Will this go on forever?
Electrochemistry
2e-
2e-
2e-
Na+
Zn electrode
Lose e- (Table J)
Oxidized
Zn+2
Cl-
2eNa+
Cl-
Cl-
Cl-
Na+
Na+
Na+
2e-
2e-
Cu electrode
Gains e- (Table J)
Cu+2
Cl-
Reduced
Cathode(+)
Anode (-)
Cl-
Zn
Zn+2
ZnSO4
Na+
2Cl-
2Na+
Cu+2
CuSO4
Cu
Half Reactions
In the reaction below, identify
what is the oxidizing agent and
the reducing agent.
Ca + H2O CaO + H2
Electrochemistry
2e-
2e-
2e-
Na+
Zn electrode
Lose e- (Table J)
Oxidized
Zn+2
Cl-
2eNa+
Cl-
Cl-
Cl-
Na+
Na+
Na+
2e-
2e-
Cu electrode
Gains e- (Table J)
Cu+2
Cl-
Reduced
Cathode(+)
Anode (-)
Cl-
Zn
Zn+2
ZnSO4
Na+
2Cl-
2Na+
Cu+2
CuSO4
Cu
Electrochemistry
Summary:
1. Voltaic Cells – are spontaneous reactions
2.
Electrons travel through the wire from more reactive
metal to the less reactive metal (Table J)
3. Salt Bridge – permits the flow of ions
4. Red Cat gets fat!
Electrochemistry
These are NOT spontaneous reactions –
they are forced by the addition of
electricity!
Occur within one container, not two
separate cells!
These reactions are used to plate metals,
purify metals and separate compounds.
Electrochemistry
Cathode
Anode
Becomes negative
Becomes positive
Picks up + ions from
solution
Gets plated with the
metal ion from the
solution
Sn
Fe
Loses positive ion (Sn+2)
to solution
During a laboratory activity, a
student reacted a piece of zinc
with 0.1M HCl(aq). Based on
Reference Table J, identify one
metal that does not react
spontaneously with HCl(aq).
Write out the oxidation and reduction half reactions for the
voltaic cell below. (Do not need drawing in notes)
NaBr
Fe electrode
K electrode
Fe
K
Fe+2
K+1
Electrochemistry
Electrochemical Cell Differences
spontaneous
non-spontaneous
Anode - negative
Anode - positive
Needs two containers
Needs one container
Packet Review
16.
NaBr
Fe electrode
K electrode
Fe
K
Fe+2
K+1
Packet Review
16.
Na2SO4
Al electrode
Ag electrode
Al
Ag
Al+3
Ag+1
Electrochemistry
Anode
Cathode
Becomes positive
Becomes negative
Picks up + ions from
solution
Loses positive ion (Cu+2)
to solution
Cu
Cu+2
Zn
Gets plated with the
metal ion from the
solution
Electrons ALWAYS flow from Anode to Cathode
Electrochemistry
And review packet
Electrochemistry
Anode
Cathode
Becomes positive
Becomes negative
Picks up + ions from
solution
Loses positive ion (Cu+2)
to solution
Cu
Cu+2
Zn
Gets plated with the
metal ion from the
solution
Electrons ALWAYS flow from Anode to Cathode
Electrochemistry
Balancing Net Ionic Equations
Done on the board
Electrochemistry
Ionic equation balancing
Electrolysis Simulation
C3H8O + CrO3 + H2SO4
Cr2(SO4)3 + C3H6O + H2O
{
Balancing equations using
oxidation numbers
BALANCING REACTIONS
-conservation of mass and charge
-we must make sure that the e- that one atom
loses must equal the e- that another atom gains
Try to balance this one:
HNO3 + I2 HIO3 + NO2 + H2O
BALANCING REACTIONS
HNO3 + I2 HIO3 + NO2 + H2O
1. Assign ox #’s, write ½ reactions and cross out spectators
N+5 + 1e- N+4
I20 - 5e- I+5
2. Balance each ½ reaction with respect to atoms and then e10 (N+5 + 1e- N+4 )
1 ( I20 - 10e- 2I+5 )
3. Distribute to all parts of the ½ reaction
10N+5 + 10e- 10N+4
I20 - 10e- 2I+5
BALANCING REACTIONS
3. Carry everything down and cross out e-
10N+5 + 10e- 10N+4
I20 - 10e- 2I+5
10N+5 + I20 10N+4 + 2I+5
4. Put coefficients back into equation and balance what is left.
10HNO3 + 1I2 10HIO3 + 2NO2 + 4 H2O
BALANCING REACTIONS
Sb + HNO3 Sb2O5 + NO + H2O
1. Assign ox #’s, write ½ reactions and cross out spectators
Sb0 Sb2+5 + 5eN+5 + 3e- N+2
2. Balance each ½ reaction with respect to atoms and then e3 ( 2Sb0 Sb2+5 + 10e- )
10 ( N+5 + 3e- N+2 )
3. Distribute to all parts of the ½ reaction
6Sb0 3Sb2+5 + 30e10N+5 + 30e- 10N+2
Most missed Part 2
Questions
1. You have a voltaic cell with copper and aluminum as the
electrodes. As the cell operates, the mass of the Al electrode
decreases. Explain, in terms of particles, why this decrease in mass
occurs.
2. Explain, in terms of electrical energy, how the operation of a
voltaic cell differs from the operation of an electrolytic cell used in the
Hall process. Include both the voltaic cell and the electrolytic cell in
your answer.
3. Explain, in terms of ions, why molten cryolite conducts electricity.
[Cryolite = Na3AlF6]
BALANCING REACTIONS
3. Carry everything down and cross out e6Sb0 3Sb2+5 + 30e10N+5 + 30e- 10N+2
6Sb0 +10N+5 3Sb2+5 + 10N+2
4. Put coefficients back into equation and balance what is left.
6Sb + 10HNO3 3Sb2O5 + 10NO + 5 H2O
Balance the following
S + HNO3 SO2 + NO + H2O
The Statue of Liberty is made of an iron
framework covered by copper metal.
Over time, a thin green layer(patina)
forms on the outside. Where the iron
came into contact with the copper a
reaction occurred where the iron was
oxidized. Why did this happen? Use
your Reference Tables.



![Redox_equations[1].](http://s2.studylib.net/store/data/005611618_1-b55ee2e3a52621e48ab97cc179174553-300x300.png)