salt water chemistry
advertisement
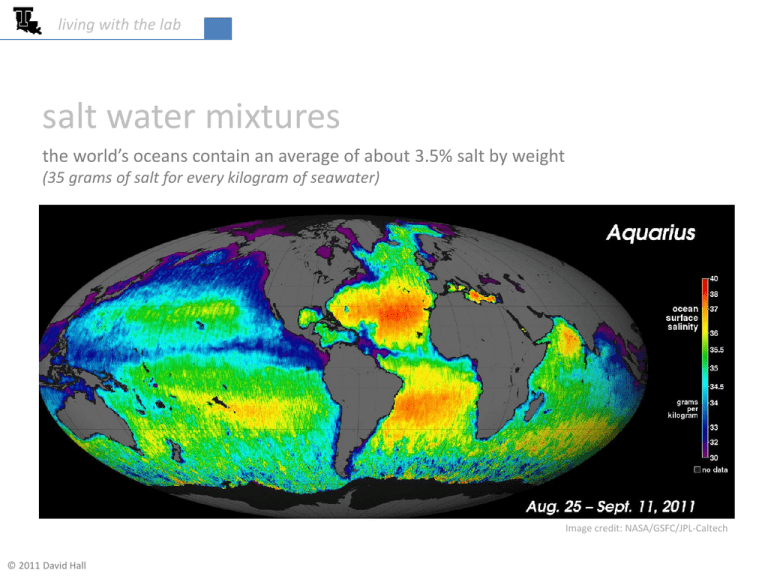
living with the lab salt water mixtures the world’s oceans contain an average of about 3.5% salt by weight (35 grams of salt for every kilogram of seawater) Image credit: NASA/GSFC/JPL-Caltech © 2011 David Hall living with the lab concentrations used for fishtank project we will calibrate our salinity sensor using small NaCl concentrations • 0.00% weight NaCl – we will actually use deionized water (DI water) • 0.05% weight NaCl • 0.10% weight NaCl • 0.15% weight NaCl 2 living with the lab why not just use tap water? • standard tap water contains dissolved ions such as sodium, calcium, iron, copper and bromide • the electrical resistivity of tap water can vary widely (15kΩ-cm is typical) • ions have been removed from DI water resulting in a much higher resistivity (18MΩ-cm is typical) Did you notice the strange units on resistivity (Ω-cm)? The electrical resistance R of a body (measured in Ω) is related to the electrical resistivity ρ (measured in Ω-cm) as 𝑳 𝑨 𝑅= 𝜌∙𝐿 𝐴 A = cross sectional area L = length over which resistance is measured ρ = resistivity which is a material property 3 living with the lab 𝑤𝑡% 𝑁𝑎𝐶𝑙 = 𝑊𝑁𝑎𝐶𝑙 𝑚𝑁𝑎𝐶𝑙 ∙ 100% = ∙ 100% 𝑊𝑁𝑎𝐶𝑙 + 𝑊𝐻2 𝑂 𝑚𝑁𝑎𝐶𝑙 + 𝑚𝐻2 𝑂 calculating percent weight A mixture contains 19 grams of water and 1 gram of NaCl. What is the weight percent of NaCl? 𝑤𝑡 % 𝑁𝑎𝐶𝑙 = 𝑊𝑁𝑎𝐶𝑙 𝑤𝑒𝑖𝑔ℎ𝑡 𝑜𝑓 𝑁𝑎𝐶𝑙 ∙ 100% ∙ 100% = 𝑊𝑁𝑎𝐶𝑙 + 𝑊𝐻2 𝑂 𝑤𝑒𝑖𝑔ℎ𝑡 𝑜𝑓 𝑚𝑖𝑥𝑡𝑢𝑟𝑒 = 𝑚𝑁𝑎𝐶𝑙 ∙ 𝑔𝑟𝑎𝑣𝑖𝑡𝑦 ∙ 100% 𝑚𝑁𝑎𝐶𝑙 ∙ 𝑔𝑟𝑎𝑣𝑖𝑡𝑦 + 𝑚𝐻2 𝑂 ∙ 𝑔𝑟𝑎𝑣𝑖𝑡𝑦 = 𝑚𝑁𝑎𝐶𝑙 ∙ 100% 𝑚𝑁𝑎𝐶𝑙 + 𝑚𝐻2 𝑂 = 1 𝑔𝑟𝑎𝑚 1 𝑔𝑟𝑎𝑚 ∙ 100% ∙ 100% = 20 𝑔𝑟𝑎𝑚𝑠 1 𝑔𝑟𝑎𝑚 + 19 𝑔𝑟𝑎𝑚𝑠 𝑔𝑟𝑎𝑣𝑖𝑡𝑦 = 9.81 𝑚 𝑠2 𝑤𝑡 % 𝑁𝑎𝐶𝑙 = 5% 4 living with the lab Class Problem If you add 9.5 grams of NaCl to 5 gallons of water, what weight percent of salt will the mixture contain? Useful conversion factors: Density of water = 𝜌𝐻2 𝑂 = 1000 kg/m3 = 1 g/cm3 = 1 kg/L at 4oC (maximum density) 1 cm3 = 1 cc = 1 ml 1 L = 0.001 m3 1 gallon = 3.7853 L 5 living with the lab Example How much salt would you need to add to 2L of water to have a concentration of 3.5 wt% NaCl? unknown 𝑤𝑡% 𝑁𝑎𝐶𝑙 = 3.5% 𝑚𝑁𝑎𝐶𝑙 ∙ 100% 𝑚𝑁𝑎𝐶𝑙 + 𝑚𝐻2 𝑂 unknown 3.5% = 𝑚𝐻2 𝑂 = 2𝐿 ∙ 1𝑘𝑔 = 2𝑘𝑔 𝐿 mass of 2L of water 𝑚𝑁𝑎𝐶𝑙 ∙ 100% 𝑚𝑁𝑎𝐶𝑙 + 2𝑘𝑔 0.035 𝑚𝑁𝑎𝐶𝑙 + 2𝑘𝑔 = 𝑚𝑁𝑎𝐶𝑙 0.035 ∙ 𝑚𝑁𝑎𝐶𝑙 +0.07𝑘𝑔 = 𝑚𝑁𝑎𝐶𝑙 1 − 0.035 ∙ 𝑚𝑁𝑎𝐶𝑙 = 0.07𝑘𝑔 𝑚𝑁𝑎𝐶𝑙 = 0.07𝑘𝑔 = 0.0725𝑘𝑔 = 72.5𝑔 1 − 0.035 6 living with the lab recall the reactions at the electrodes 5V e- e- 10 kΩ anode – oxidation cathode – reduction (loss of electrons) e- (gain of electrons) e- Cl- Cl- Na+ Cl2 Cl- Na+ Cl- Cl Cl- ClCl- Na+ Na+ Cl Cl- reduction occurs at the negatively charged cathode: Na+ Na+ Na+ Cl- Na+ ion migration OHee- 2 𝐻2 𝑂(𝑙) + 2𝑒 − → 𝐻2 𝑔 + 2𝑂𝐻− (𝑎𝑞) H H2O H H2O OH- ClNa+ Cl- Cl- Na+ Na+ Na+ Na+ is a spectator ion oxidation occurs at the positively charged anode: 2 𝐶𝑙 − 𝑎𝑞 → 𝐶𝑙2 𝑔 + 2𝑒 − 7 living with the lab useful information for reaction calculations 𝑔 Atomic Weight of Na = 22.99 𝑚𝑜𝑙 Atomic Weight of Cl = 35.45 𝑔 𝑚𝑜𝑙 𝑔 Atomic Weight of NaCl = 58.44 𝑚𝑜𝑙 Avogadro’s Number = 6.022 x 1023 𝑚𝑜𝑙 1 Coulomb = 6.24 x 1018 electrons 8 living with the lab 𝑔 Atomic Weight of NaCl = 58.44 𝑚𝑜𝑙 Avogadro’s Number = 6.022 10 23 𝑚𝑜𝑙 Class Problem Assume you have 5 gallons of water to which you add salt to create a mixture with 0.2 wt% NaCl. Determine: (a) the mass of the water (b) the mass of the salt (c) the number of moles of NaCl (d) the number of Cl- ions 9 living with the lab 1 Coulomb = 6.24 x 1018 electrons 1A= 1 𝐶𝑜𝑢𝑙𝑜𝑚𝑏 𝑠 Example If a constant current of 0.1mA passes through the probes of the conductivity sensor, how many H2 gas molecules would be formed over a 1 minute period? HINT: Use the definition of an amp and a Coulomb along with the chemical reaction at the cathode. 2 𝐻2 𝑂(𝑙) + 2𝑒 − → 𝐻2 𝑔 + 2𝑂𝐻− (𝑎𝑞) two electrons pass through the circuit for each H2 gas molecule formed First, find the number of electrons that pass through the sensor per second. 𝑒− 𝐴 = 0.1𝑚𝐴 ∙ ∙ 𝑠 1000 𝑚𝐴 𝐶𝑜𝑢𝑙𝑜𝑚𝑏 6.24 10 18 𝑒 − 𝑠 = 6.24 10 ∙ 𝐴 𝐶𝑜𝑢𝑙𝑜𝑚𝑏 14 𝑒− 𝑠 Now, find the number of H2 molecules over a 1 minute period. 𝐻2 𝑚𝑜𝑙𝑒𝑐𝑢𝑙𝑒𝑠 = 6.24 10 14 1 𝐻2 𝑚𝑜𝑙𝑒𝑐𝑢𝑙𝑒 𝑒 − 60𝑠 ∙ ∙ 1 𝑚𝑖𝑛 ∙ = 1.87 10 2 𝑒− 𝑠 𝑚𝑖𝑛 16 𝐻2 𝑚𝑜𝑙𝑒𝑐𝑢𝑙𝑒𝑠 10