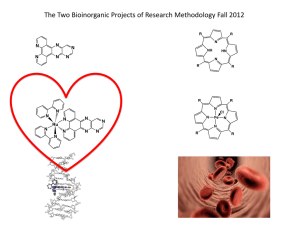
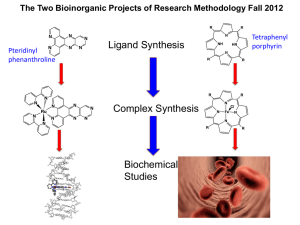
The *Blue* Dimer: Water Oxidation Catalyst
advertisement

THE “BLUE” DIMER: WATER
OXIDATION CATALYST
Presented By: Margo Roemeling
Mentor: Dr. James K. Hurst
THE BASIS FOR LIFE
Photosynthesis is the basis for all aerobic
life on Earth.
The process uses water and carbon
dioxide as a source of electrons to make
sugar and oxygen as a bi-product.
It involves the use of biocatalysts and
energy from sunlight.
PHOTOSYNTHESIS AND THE OXYGEN
EVOLVING COMPLEX
Electron
Acceptor
Pigment
s
Oxygen
Evolving
Complex
(O.E.C.)
OXYGEN EVOLVING COMPLEX MECHANISM
The O.E.C. is the water
oxidation center of PSII
Has a 4 Mn metal
active center
Di-oxo bridges
2 terminal waters
Uses 4 photons to lose 4
e- and 4 H+ from waters
Upon reaching the S4
state, O2 is given off
and 2 waters are taken
up to bring it back to its
most reduced state.
Light is flashed on the photo reaction center, and
every four flashes, O2 is given off.
“ARTIFICIAL BIOSYNTHESIS”
“Artificial biosynthesis” attempts to mimic
photosynthetic reactions in simpler systems
In the growing energy crisis, it has become exceedingly
important that we find new alternative fuels to replace
fossil fuels.
Using these systems, we could mimic the way
photosynthesis converts sunlight to energy, and
convert sunlight into usable fuel energy.
Artificial biosynthesis could yield hydrogen fuel as well
as alcohol fuels.
A SIMPLER SYSTEM
hn
2H2O
2H2 (or CH2O + H2O)
WOC
O2 + 4H+
4[e-]
photoactive
element
4H+ (+ CO2)
THE “BLUE DIMER”
The “blue dimer” is a very effective catalyst for
water oxidation (like the O.E.C.)
Water oxidation mechanisms of blue dimer are
analogous to the water oxidation mechanisms in the
O.E.C.
STRUCTURE
2 Ruthenium
metal centers
2 Terminal
waters
Oxygen
3 Bipyridine
ligands
“BLUE DIMER” VS. O.E.C.
{3,3}-[(bpy)2Ru(OH2)]2O4+
PSII
“BLUE DIMER” MECHANISM
2 Ru metal active
{5,5}
center
Mono-oxo bridge
2 terminal waters
e,H
+
Loses 4 e- and 4 H
from water
Upon reaching {5,5}
oxidation state, gives off
an O2 and takes up 2
waters bringing it back
to its most reduced
state.
{3,3}
e-,
H+
-
{3,4}
+
{4,5}
{4,4}
e-, H+
e-, H+
THE CRUCIAL STEP
•The two ruthenyl groups are structurally situated to allow
joint addition of water to form the peroxo-bound intermediate
•Once formed, the intermediate species is unstable and
internal electronic rearrangements lead directly to the final
products ({3,3} and O2).
4+
{5,5}
(bpy) 2 Ru
O
O
H
O
Ru(bpy)2
(bpy)2 Ru
O
O
O
H2O
H
4+
{3,3}
(bpy)2 Ru
OH2
O
Ru(bpy) 2
OH2
4+
{4,4}
O-O
Ru(bpy) 2
OH
OH
A SIMILAR SYSTEM
“Pigment”, Photoreaction center
Water Oxidation Center
Electron acceptor
THE REACTION: PERSULFATE
S2O8 is often used as a reactant (electron
acceptor) to study catalyzed water oxidation by
redox-active metal ions.
S2O822SO42
SURPRISING RESULTS
Persulfate reacts thermally with the blue dimer
and partially oxidizes it.
2{3,3} + S2O822{3,4} + 2SO42 We need to understand this reaction and its
relationship to the overall photocatalytic system.
QUESTION
Does this reaction involve direct reaction between
persulfate and {3,3}?
S2O82- + {3,3}
(2) SO4.- + {3,3} fast
Net: S2O8 + 2{3,3}
(1)
{3,4} + SO42- + SO4.2{3,4} + 2SO422{3,4} + 2SO42-
Or is it indirect?
S2O82-
2SO4.-
SO4
fast
.- + {3,3}
{3,4} + SO42-
HYPOTHESIS
We can use kinetics to distinguish between these
reactions.
If direct,
Rate = K[S2O8][{3,3}]
If indirect,
Rate = K[S2O8]
METHODS
To study the kinetics of the
reaction, a special
instrument is used.
Because the reactions are
very fast in basic solution,
we use a stopped-flow
machine.
2 Syringes, one for each
solution.
Solutions are quickly
mixed and absorption is
measured for 100 seconds
as reaction is progressing.
STOPPED-FLOW TRACE
•This trace
from the
stopped-flow
machine
shows the
exponential
decay of the
reaction
which tells us
that it is first
order.
PH
DEPENDENCE
The reaction is
very fast in basic
solution and
very slow in
acidic solution.
THE RATE LAW
From the stopped-flow data, we can get the
rate law.
If Rate = k[{3,3}][S2O8], this means that it is
first order in {3,3}
So, we can treat [S2O8] as a constant making
the new rate law: Rate = k’[{3,3}]
FIRST ORDER REACTION
•Using Rate = k’[{3,3}],
we can graph k’ vs.
[S2O8]
5/8/2010
0.25
2-
{3,3} + S2O8 in
2 mM borate, pH 9.2
k (s-1)
0.20
•And if the reaction is
first order in {3,3} like we
predicted, we should see
a straight line.
0.15
0.10
0.05
0.00
0
100
200
300
[S2O82-] (M)
400
500
FURTHER TESTS
•To further test our
hypothesis of direct
reaction, we tested the
reaction at various
ionic strengths.
•If there was direct
reaction between S2O8
and {3,3} we would see
that as ionic strength
increases, the rate of
the reaction decreases.
TEMPERATURE DEPENDENCE
•Testing the
temperature
dependence
allowed us to look
at the activation
energy barrier of
the reaction
FUTURE
In the future, we plan to use computer modeling
simulations to further study the kinetics of the
reaction.
ACKNOWLEDGEMENTS
Howard Hughes Medical Institute
Dr. James K. Hurst
Dr. Kevin Ahern
The Beckman Lab Group