Chapter 23: Catalysis
advertisement

Atkins & de Paula:
Atkins’ Physical Chemistry 9e
Chapter 23: Catalysis
Chapter 23: Catalysis
catalyst, a substance that accelerates a reaction but undergoes no net chemical change.
enzyme, a biological catalyst.
homogeneous catalyst, a catalyst in the same phase as the reaction mixture.
heterogeneous catalyst, a catalyst in a different phase from the reaction mixture.
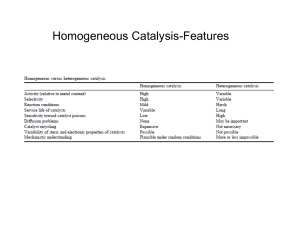
HOMOGENEOUS CATALYSIS
23.1 Features of homogeneous catalysis
acid catalysis, catalysis by the transfer of a proton from an acid to the substrate.
base catalysis, catalysis by the transfer of a proton from the substrate to a base.
Chapter 23: Catalysis
23.2 Enzymes
active site, the region of an enzyme molecule at which reaction takes place.
substrate, the species that reacts in the presence of an enzyme.
lock-and-key model, a model of enzyme action in which the substrate and the active
site have complementary shapes.
induced-fit model, a variation of the lock-and-key model in which the substrate causes
a conformational change in the active site.
Chapter 23: Catalysis
23.2(a) The Michaelis-Menten mechanism of enzyme catalysis
Michaelis–Menten mechanism, a mechanism for enzyme-catalysed reactions.
E S ES
k a , k a
ES P E
kb
v kb [ES]
ka
d [ES]
[E][S]
k a [E][S] k a [ES] kb [ES] 0 [ES]
dt
k a kb
k a kb [E][S]
KM
; Michaelisconstant
ka
[ES]
[E]0 [E] [ES], [S] [S] 0 [ES]
v
[E]0
1 K M /[S] 0
kb [E]0
; Michaelis- Mentenequation
1 K M /[S] 0
Chapter 23: Catalysis
23.2(a) The Michaelis-Menten mechanism of enzyme catalysis
maximum velocity, the greatest reaction rate for a given concentration of substrate.
v
kb [E]0
1 K M /[S] 0
[S] 0 K M : v
kb
[S] 0 [E]0
KM
[S] 0 K M : v vmax kb [E]0
v
kb [E]0
vmax
1 K M /[S] 0
1 K M /[S] 0
1
1 KM 1
; Lineweaver- Burk plot
v vmax vmax [S] 0
Chapter 23: Catalysis
23.2(b) The catalytic efficiency of enzymes
turnover number (or catalytic constant), the number of catalytic cycles (turnovers)
performed by the active site in a given interval divided by the duration of the interval;
kcat = kb = vmax/[E]0.
catalytic efficiency, η = kcat/KM = kakb/(ka + kb).
Excersize Example 23.1
Chapter 23: Catalysis
23.2(c) Mechanism of enzyme inhibition
E S ES
k a , k a
ES P E
kb
[E][I]
[EI]
[ES][I]
ESI ES I K I
[ESI ]
EI E I
KI
[E]0 [E] [EI] [ES] [ESI ]
1
[I]
KI
and
1
[I]
K I
[E]0 [E] [ES]
KM
K
[E][S]
K [ES]
, [S] [S] 0 [E]0 M
[ES] [ES] M
[ES]
[S] 0
[S] 0
v kb [ES]
kb [E]0
vmax
K M /[S]0 K M /[S]0
1 K M 1
v vmax vmax [S] 0
Chapter 23: Catalysis
23.2(c) Mechanism of enzyme inhibition
competitive inhibition, inhibition in which the
inhibitor binds only to the active site of the enzyme;
α > 1 and α = 1.
uncompetitive inhibition, inhibition in which the
inhibitor binds to a site of the enzyme that is
removed from the active site, but only if the substrate
is already present; α = 1 and α > 1.
non-competitive inhibition (or mixed inhibition),
inhibition in which the inhibitor binds to a site other
than the active site; α > 1 and α > 1.
Excersize Example 23.2
Chapter 23: Catalysis
HETEROGENEOUS CATALYSIS
23.3 THE GROWTH AND STRUCTURE OF SOLID SURFACES
adsorption, the attachment of particles to a surface .
adsorbate, the substance adsorbed.
adsorbent (or substrate), the substance on which another substance in adsorbed.
desorption, the detachment of an adsorbed substance.
23.3(a) Surface growth
step, a discontinuity between two otherwise flat layers.
terrace, a flat region of a surface.
Chapter 23: Catalysis
23.3(b) Surface composition and structure
ultrahigh vacuum (UHV), pressures lower than about 10–7 Pa.
photoemission spectroscopy, photoelectron spectroscopy applied to surfaces.
Auger electron spectroscopy (AES), spectroscopy based on the Auger effect.
Auger effect, the emission of a second electron after high energy radiation has expelled
another.
X-ray fluorescence, the generation of fluorescence by the Auger effect.
scanning Auger electron microscopy (SAM), a technique for mapping the spatial
variation over a surface.
XPS
X-ray
fluorescence
AES
Chapter 23: Catalysis
reconstruction, modification of the substrate surface layers in response to adsorbates.
low-energy electron diffraction (LEED), electron diffraction by surfaces.
Chapter 23: Catalysis
electron energy loss spectroscopy (EELS or HREELS), a technique in which the
energy loss suffered by a beam of electrons is monitored when they are reflected from a
surface.
reflection–absorption infrared spectroscopy (RAIRS), a technique for obtaining the
infrared absorption spectrum of the adsorbate.
surface-enhanced Raman scattering (SERS), strong enhancement of the Raman
spectrum of the adsorbate.
surface-extended X-ray absorption fine structure spectroscopy (SEXAFS),
spectroscopy that makes use of the oscillations in X-ray absorbance observed on the
high-frequency side of an absorption edge.
molecular beam scattering (MBS), the scattering of a beam of adsorbate molecules by
a surface.
Chapter 23: Catalysis
23.4 THE EXTENT OF ADSORPTION
fractional coverage, θ, the fraction of adsorption sites occupied.
rate of adsorption, the rate of change of fractional coverage; dθ/dt.
flash desorption, a technique in which a sample is suddenly heated and the resulting rise
of pressure is interpreted in terms of the amount of adsorbate originally on the sample.
gravimetry, the determination of fractional coverage by measurement of mass.
quartz crystal microbalance (QCM), the determination of mass that makes use of the
modification of the crystal’s vibrational frequency by an adsorbate.
23.4(a) Physisorption and chemisorption
physisorption, adsorption by van der Waals interaction between the adsorbate and the
substrate.
chemisorption, adsorption by the formation of a chemical bond.
Chapter 23: Catalysis
23.4(b) Adsorption isotherms
adsorption isotherm, the relation between fractional coverage and partial pressure of a
substrate.
Langmuir isotherm; based on the 3 assumptions.
1) No adsorption beyond monolayer
2) All surface sites are equivalent.
3) Adsorption does not depend on the coverage (no interaction between adsorbates)
A(g) M(surface) AM(surface)
ka , kd
d
k a pN (1 ) k d N 0 at equilibrium
dt
k
Kp
K a
1 Kp
kd
For adsorptionwith dissociation,
d
k a p{N (1 )}2 k d ( N ) 2 0 at equilibrium
dt
( Kp )1/ 2
1 ( Kp )1/ 2
Excersize
Example 23.4,5
Chapter 23: Catalysis
isosteric enthalpy of adsorption, the standard enthalpy of adsorption at a fixed surface
coverage; ΔadHθ =RT2( ln K/T)θ.
BET isotherm, V/Vmon = cz/(1 – z){1 – (1 – c)z}, z = p/p*.
Temkin isotherm, θ = c1 ln(c2p).
Freundlich isotherm, θ = c1p1/c2.
BET isotherm
Excersize
Example 23.6
Chapter 23: Catalysis
23.5 The rates of surface processes
second harmonic generation (SHG), the process of
generating radiation of twice the incident frequency (by a
surface layer).
precursor state, the initial state of an adsorbate
molecule on a surface before it forms a chemical bond.
23.5(a) The rate of adsorption
sticking probability, s, the proportion of collisions with
a surface that lead to adsorption; s = (1 – θ)s0.
Chapter 23: Catalysis
23.5(b) The rate of desorption
half-life for adsorption, t1/2 = (ln 2)/kd (kd =Ae-Ed/RT).
temperature-programmed desorption (TPD), the observation of a surge in desorption
rate when the temperature is raised linearly.
thermal desorption spectroscopy (TDS), anther name for temperature-programmed
desorption.
23.5(c) Mobility on surfaces
field-ionization microscopy (FIM), a technique that portrays the electrical characteristics
of a surface by using the ionization of noble gas atoms.
Chapter 23: Catalysis
23.6 Mechanisms of heterogeneous catalysis
co-adsorption, the joint adsorption of two or more adsorbates.
Langmuir–Hinshelwood mechanism, a reaction that takes place by encounters between
molecular fragments and atoms adsorbed on the surface.
ABP
A
v kr A B
K A pA
K B pB
kr K A K B pA pB
, B
v
1 K A pA K B pB
1 K A pA K B pB
(1 K A pA K B pB ) 2
Eley–Rideal mechanism, a reaction in which a gas–phase molecule collides with another
molecule already adsorbed on the surface.
ABP
v
v kr pB A
kr KpA pB
1 KpA
KpA 1, v kr pB
KpA 1, v kr KpA pB
Chapter 23: Catalysis
23.7 Catalytic activity at surfaces
molecular beam reactive scattering (MBRS), reactive scattering between a molecular
beam and adsorbed molecules.
pulsed beams, a technique in which a molecular beam is chopped into short slugs.