Measurement of Binding Constants and Heats of
Binding using Isothermal Titration Calorimeter
Wei Li
Department of Chemistry
University of Victoria
Winter, 2013
Acknowledgement
Professor Hof
Classmates
Outline
•
•
•
•
•
•
•
•
•
Thermodynamic parameters
Isothermal titration calorimeter (ITC)
ITC development history
Advantages of using ITC
Basic configuration of an ITC
Sample preparation
Raw data and plot of processed data
Systematic errors
References
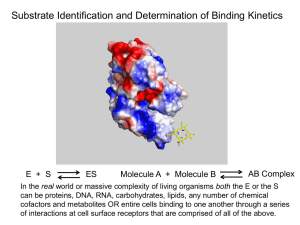
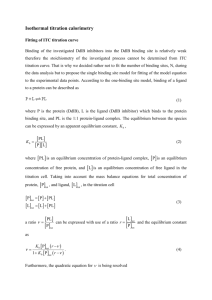
Thermodynamic Parameters
When characterizing interactions between a biological macromolecule M and a
small ligand X, or between two macromolecules,
M +X
= MX
MX + X = MX2
…….
MXn-1 + X = MXn,
The single-site binding constant K, H°,
and the number of sites n are the
independent variables of thermodynamic
interest.
G° and S° of binding are dependent
variables obtained by the calculation.
K = [MXn]/[MXn-1] X]
G = G° + RTlnK (Standard conditions: 1 mole of P and 1 mole of L at PH7 and 25° C)
At equilibrium, under standard conditions, G = 0.
G° = -RTlnK = H° - TS°
Thermodynamic Parameters
Entropy
• Hydrophobic interactions
• Water release
• Ion release
• Conformational changes
Enthalpy
• Hydrogen bonding
• Protonation events
n = stoichiometry
Number of protein binding sites reflects
the purity and the functional integrity of a
protein preparation if the measure and
fitted stoichiometry can be compared to
the
known,
previously
determined
stoichiometry
ITC Development History
•
•
•
1960s – built in the second half to study chemical reactions
1970s – sensitivity: mJ
metal + ligand complex
1980s – sensitivity: µJ
ligand binding processes and micelle formation
•
1990s – number of published papers increased due to new commercial
calorimeters became available. Evolved from a specialist method to a
widely used technique
•
2000s – widely employed in the design and discovery of new drugs and
for the study of liquid mixtures
•
2009 – 374 out of 432 papers on protein interaction with other proteins,
small molecules, metal ions, lipids, nucleic acids, and carbohydrates as
well as on nucleic acid interactions with small molecules.
Advantages of using ITC
No labeling required
In-solution
No molecular weight limitations
Optical clarity unimportant
Minimal assay development
Using heat as signal
In one single experiment, K, ∆H, and Stoichiometry n of
interaction between two or more molecules in solution can be
determined
∆G and ∆S can be calculated
Basic Configuration of an ITC
VP-ITC micro calorimeter
•
•
•
•
Reference cell and sample cell in an adiabatic jacket.
Reference cell filled with buffer or water. Sample cell filled with protein.
Both cells are heated in such a way that the temperature is almost
constant, i.e. T < 10 x (E-6) ° C at all times.
Syringe device holds the ligand. This device titrates the ligand into the
sample cell and also acts as a stirrer.
“Isothermal titration calorimetry (ITC) is a biophysical technique that allows a
thermodynamic characterization of an interactive system”.
Basic Configuration of an ITC
•
•
•
•
Reference and sample cells containing
identical buffer are located within an
adiabatic jacket, and the latter contains
macromolecule of interest.
A small, constant power is applied to the
reference cell, which activated the cell
feedback circuit to drive ∆T to 0.
No reaction – the feedback power
remains constant at the resting baseline
value.
Exothermal reactions temporarily ↓ and
endothermic reactions temporarily ↑
feedback power. The reaction heats are
readily obtained by computer integration
of these deflections from the resting
baseline.
Sample Preparation
General consideration
• Experiment performed at reasonable PH
• Protein has to be extensively dialysed and the ligand has to be dissolved
in the buffer recovered from the last protein dialysis step
Protein preparation
• As pure as possible
• Concentration should be correctly estimated.
Ligand preparation
• As pure as possible
• Accurate concentration
Buffers
Buffers with low ionization enthalpy should be considered.
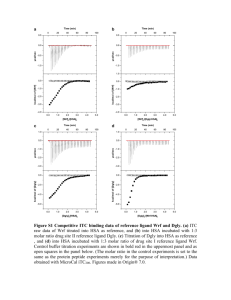
Raw Data and Plot of Processed Data
•
•
•
Top: Raw ITC data showing an
exothermic binding reaction. Each peak
corresponds to an injection of ligand into
a protein solution in the sample cell; the
area under the peak is proportional to
the amount of heat released in the
binding reaction.
When the protein becomes saturated,
the DP signal diminishes until only the
background heat of dilution is observed.
Bottom: Binding isotherm obtained by
integrating the area of each peak. The
heat released per mole of ligant is
plotted against the molar ratio of the two
reactants.
Systematic Errors
Blank experiment
Heat of dilution of ligand
Heat of dilution of macromolecule
Buffer to buffer experiment
Heat of proton ionization
Degassing
Generation of bubbles during an ITC experiment will generate spurious heat signals.
Measurements of the concentrations
Accuracy of the measurements are imperative for a good ITC experiment.
Sample concentrations also need to be within a proper range to get reliable data.
Small molecules impurities and PH mismatches in the buffer will cause artifacts.
References
•
•
•
•
•
Nunez, S.; Venhorst, J.; Kruse, C. G. Target–drug interactions: First
principles and their application to drug discovery. Drug Discov. Today
2012, 17, 10–22.
Wiseman T. et al, Rapid measurement of binding constants and heats of
binding using a new titration calorimeter. Anal Biochem 179: 131-137,
1989.
Mark A. Williams and Tina Daviter (eds), Protein-Ligand Interactions:
methods and applications. Springer Science and Business Media, New
York, 2013.
Joel Tellinghuisen, John D. Chodera, Systematic errors in isothermal
titration calorimetry: concentrations and baselines, Analytical
Biochemistry 414 (2011) 297-299.
wikepedia
Human serum albumin
Transferrin