Poster
advertisement
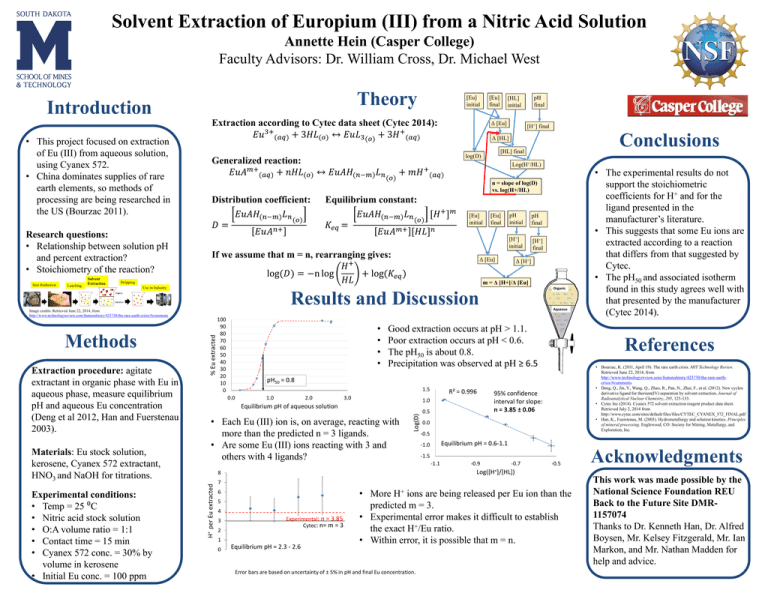
Solvent Extraction of Europium (III) from a Nitric Acid Solution Annette Hein (Casper College) Faculty Advisors: Dr. William Cross, Dr. Michael West Theory Introduction • This project focused on extraction of Eu (III) from aqueous solution, using Cyanex 572. • China dominates supplies of rare earth elements, so methods of processing are being researched in the US (Bourzac 2011). Research questions: • Relationship between solution pH and percent extraction? • Stoichiometry of the reaction? Size Reduction Leaching Solvent Extraction Stripping [Eu] initial Extraction according to Cytec data sheet (Cytec 2014): 𝐸𝑢3+ (𝑎𝑞) + 3𝐻𝐿(𝑜) ↔ 𝐸𝑢𝐿3 (𝑜) + 3𝐻 + (𝑎𝑞) Generalized reaction: 𝐸𝑢𝐴𝑚+ (𝑎𝑞) + 𝑛𝐻𝐿(𝑜) ↔ 𝐸𝑢𝐴𝐻(𝑛−𝑚) 𝐿𝑛 Distribution coefficient: 𝐸𝑢𝐴𝐻(𝑛−𝑚) 𝐿𝑛 (𝑜) 𝐷= 𝐸𝑢𝐴𝑛+ [Eu] final Δ [Eu] [H+] final Conclusions Δ [HL] [HL] final log(D) (𝑜) pH final [HL] initial Log(H+/HL) + 𝑚𝐻 + (𝑎𝑞) n = slope of log(D) vs. log(H+/HL) Equilibrium constant: + 𝑚 𝐸𝑢𝐴𝐻(𝑛−𝑚) 𝐿𝑛 [𝐻 ] (𝑜) 𝐾𝑒𝑞 = 𝐸𝑢𝐴𝑚+ [𝐻𝐿]𝑛 [Eu] initial If we assume that m = n, rearranging gives: 𝐻+ log(𝐷) = −n log + log(𝐾𝑒𝑞 ) 𝐻𝐿 [Eu] final pH initial pH final [H+] initial [H+] final Δ [Eu] Δ [H+] m = Δ [H+]/Δ [Eu] Use in Industry Organic Results and Discussion Materials: Eu stock solution, kerosene, Cyanex 572 extractant, HNO3 and NaOH for titrations. Experimental conditions: • Temp = 25 ⁰C • Nitric acid stock solution • O:A volume ratio = 1:1 • Contact time = 15 min • Cyanex 572 conc. = 30% by volume in kerosene • Initial Eu conc. = 100 ppm 100 90 80 70 60 50 40 30 20 10 0 • • • • Aqueous Good extraction occurs at pH > 1.1. Poor extraction occurs at pH < 0.6. The pH50 is about 0.8. Precipitation was observed at pH ≥ 6.5 References • Bourzac, K. (2011, April 19). The rare earth crisis. MIT Technology Review. Retrieved June 22, 2014, from http://www.technologyreview.com/featuredstory/423730/the-rare-earthcrisis/#comments • Deng, Q., Jin, Y., Wang, Q., Zhao, R., Pan, N., Zhai, F., et al. (2012). New cyclen derivative ligand for thorium(IV) separation by solvent extraction. Journal of Radioanalytical Nuclear Chemistry, 295, 125-133. • Cytec Inc (2014). Cyanex 572 solvent extraction reagent product data sheet. Retrieved July 2, 2014 from http://www.cytec.com/sites/default/files/files/CYTEC_CYANEX_572_FINAL.pdf • Han, K., Fuerstenau, M. (2003). Hydrometallurgy and solution kinetics. Principles of mineral processing. Englewood, CO: Society for Mining, Metallurgy, and Exploration, Inc. pH50 ≈ 0.8 1.5 0.0 1.0 2.0 3.0 R² = 0.996 1.0 Equilibrium pH of aqueous solution • Each Eu (III) ion is, on average, reacting with more than the predicted n = 3 ligands. • Are some Eu (III) ions reacting with 3 and others with 4 ligands? Log(D) Extraction procedure: agitate extractant in organic phase with Eu in aqueous phase, measure equilibrium pH and aqueous Eu concentration (Deng et al 2012, Han and Fuerstenau 2003). 0.5 95% confidence interval for slope: n = 3.85 ± 0.06 0.0 -0.5 Equilibrium pH = 0.6-1.1 -1.0 -1.5 -1.1 -0.9 -0.7 -0.5 7 6 5 4 3 2 Experimental: n = 3.85 Cytec: n= m = 3 1 0 Acknowledgments Log([H+]/[HL]) 8 H+ per Eu extracted Methods % Eu extracted Image credits: Retrieved June 22, 2014, from http://www.technologyreview.com/featuredstory/423730/the-rare-earth-crisis/#comments • The experimental results do not support the stoichiometric coefficients for H+ and for the ligand presented in the manufacturer’s literature. • This suggests that some Eu ions are extracted according to a reaction that differs from that suggested by Cytec. • The pH50 and associated isotherm found in this study agrees well with that presented by the manufacturer (Cytec 2014). Equilibrium pH = 2.3 - 2.6 • More H+ ions are being released per Eu ion than the predicted m = 3. • Experimental error makes it difficult to establish the exact H+/Eu ratio. • Within error, it is possible that m = n. Error bars are based on uncertainty of ± 5% in pH and final Eu concentration. This work was made possible by the National Science Foundation REU Back to the Future Site DMR1157074 Thanks to Dr. Kenneth Han, Dr. Alfred Boysen, Mr. Kelsey Fitzgerald, Mr. Ian Markon, and Mr. Nathan Madden for help and advice.