Why shall we enrich proteins
with specific isotopes?
Structural determination through NMR
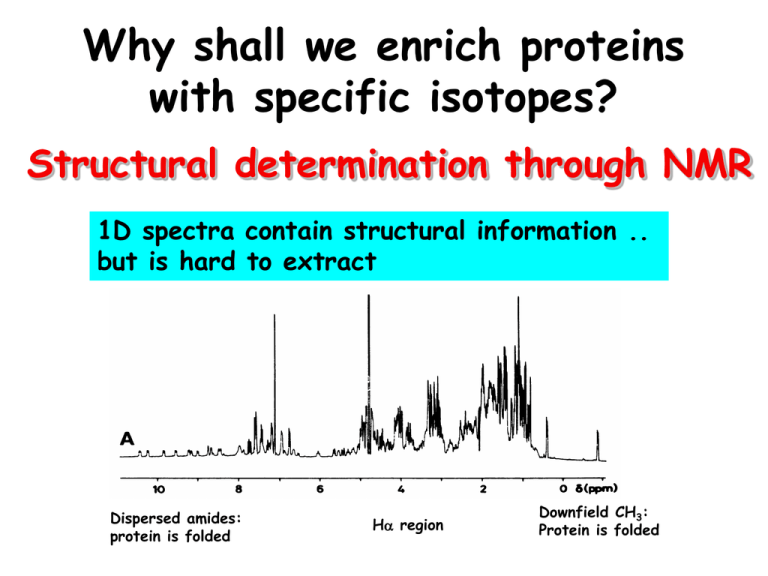
1D spectra contain structural information ..
but is hard to extract
Dispersed amides:
protein is folded
Ha region
Downfield CH3:
Protein is folded
Why shall we enrich proteins
with specific isotopes?
Even 2D spectra can be (and indeed are) very crowded
1H
1H
Realistic limit of homonuclear NMR: proteins of 100-120 amino acids;
spectra of larger proteins are too crowded
Useful nuclei such as
are rare
Isotope Spin Natural
(I) abundance
1H
1/2
2H
1
13C
1/2
14N
1
15N
1/2
17O
5/2
19F
1/2
23Na 3/2
31P
1/2
113Cd 1/2
99.985 %
0.015
1.108
99.63
0.37
0.037
100
100
100
12.26
15N, 13C
Magnetogyric ratio
g/107 rad T-1s-1
26.7519
4.1066
6.7283
1.9338
-2.712
-3.6279
25.181
7.08013
10.841
-5.9550
NMR frequency
MHz
(2.3 T magnet)
100.000000
15.351
25.145
7.228
10.136783
13.561
94.094003
26.466
40.480737
22.193173
The solution is…
The solution is
3D heteronuclear NMR
Isotopic labeling
Requirements for heteronuclear
NMR: isotope labeling
Uniform
labeling
Isotopically labeled proteins can be prepared
straightforwardly in E. coli by growing cells in minimal
media (e.g. M9) supplemented with appropriate nutrients
(15NH4Cl, 13C-glucose) or in labelled media.
Residue
specific labeling
Metabolic pathways can be exploited and/or appropriate
auxotrophic strains of E. coli can also be used for residue
selective labeling
Requirements for heteronuclear
NMR: isotope labeling
Deuterium
labeling
For large proteins deuterium labeling provides simplified spectra
for the remaining 1H nuclei and has useful effects on relaxation
properties of attached or adjacent atoms (1H, 15N,13C).
-Fractional and complete deuteration
Labeling
in eukaryotic organisms
Eukaryotic proteins which are inefficiently expressed in
bacteria can be efficiently expressed and labelled in yeast
strains (P. pastoris)
Isotopic Uniform Labelling of proteins
15N-labelled
Protein Preparation
The protein is produced by expression from bacteria which are grown on minimal
medium supplemented with 15NH4Cl and wild-type (wt) glucose.
15N, 13C-labelled
Protein Preparation
15N,13C-labelling is commonly referred to as double labelling. The protein is
produced by expression from bacteria which are grown on minimal medium
supplemented with 15NH4Cl and 13C-glucose.
15N, 13C,2H-labelled
Protein Preparation
15N,13C,2H-labelling is commonly referred to as triple labelling. The protein is
produced by expression from bacteria which are grown on minimal medium
supplemented with 15NH4Cl and 13C-glucose and using D2O instead of H2O. This
will result in about 70-80% deuteration of the side-chains, as there is a certain
amount of contaminating 1H present from the glucose. Higher levels of deuteration
of around 95% can be achieved if 13C,2H-glucose is used. However, this is more
expensive and in many cases the cheaper version is sufficient. Note that the NH
groups are exchangeable. This means that they will back-exchange to 1H when the
protein is purified in normal aqueous solution. In this way, many of the normal NHbased experiments can be carried out on triple-labelled protein.
Sources of isotopes used for uniform labeling
In most cases (except in cell-free labelling), the protein is expressed by bacteria.
The isotopic labels are introduced by feeding the bacteria specific nutrients. In most
cases the basis will be so-called minimal medium which contains all the salts and
trace elements needed by the bacteria but contains no carbon or nitrogen sources.
These elements can then be introduced using a variety of different isotopically
labelled carbon and nitrogen sources.
The simplest labeling, and also the cheapest one is 15N, because 15N ammonia is
quite cheap.
13C
is more expensive, because it requires the synthesis (most commonly biosynthesis) of 13C glucose or glycerol.
Some expression systems allow use of cheaper 13CO2.
A standard protocol for
isotope labeling
+ IPTG
Induction
O/N Inoculum in
unlabeled medium
Massive culture in
labeled medium
Harvesting
From protein purification to
check folding
Protein isolation and purification
Protein isolation and purification will follow the standard
procedure which has been set up for unlabelled protein
Check folding
Protein folding can be checked by 1H NMR and 1H-15N HSQC
spectra.
How to optimize protein
expression?
Choice of culture medium
Two main types of culture media can be tested for uniform labeling:
Ready-to-use media like algae or bacteria hydrolysate
Minimal media added with 15N nitrogen source or/and 13C
carbon source
Minimal media
Minimal media are composed in the lab and are made of nutrients
like C and N source, salts, buffering substances, traces elements
and vitamins.
Carbon source can be glucose (the best as gives highest yields),
glycerol, acetate, succinate, methanol, Etc.
In case of 13C labeling the concentration of carbon source
can be reduced with respect to unlabelled culture, to reduce costs!!!
Checks must be performed before labelling!
Nitrogen source can be NH4Cl or (NH4) 2SO4
In case of 15N labeling the concentration of nitrogen source
can be reduced with respect to unlabeled culture, to reduce costs!!!
Checks must be performed before labeling!
Minimal media
Minimal media are composed in the lab and are made of nutrients
like C and N source, salts, buffering substances, traces elements
and vitamins.
Salts are NaCl/KCl, MgSO4, CaCl2
Buffer usually is phosphate, pH 7.5
Trace elements is constituted by a mixtures of metal ions,
like Co2+, Cu2+, Zn2+, Mn2+, Fe2+
Vitamins are thiamine, biotin, folic acid, niacinamide, pantothenic
acid, pyridoxal, riboflavin
Ready-to-use media
These media are usually sterile and in the correct dilution
They can be used for massive culture in the same way as unlabeled,
rich media like LB or 2 x YT.
Some media yield predictable cell densities
Comparison between mineral
and ready-to-use media
Bacterial growth is
usually higher in
ready-to-use media
than in minimal media.
Comparison between mineral
and ready-to-use media
But protein expression?
It must be tested, case by case, through expression tests
Example:
Strategies to improve
protein expression
An example:
Grow cell mass on unlabeled rich media allowing
rapid growth to high cell density.
Exchange the cell into a labeled medium at
higher cell densities optimized for maximal protein
expression
Marley J et al. J. Biomol. NMR 2001, 20, 71-75
Strategies to improve
protein expression
In practice:
Cells are grown in rich unlabeled medium.
When OD600 = 0.7 cells are harvested, washed with
M9 salt solution, w.o. N and C source and resuspended
in labeled media at a higher cell concentration.
Protein expression is induced after 1 hour by addition of
IPTG.
The need of deuteration
Why is necessary to enrich the protein with 2H?
Deuteration reduces the relaxation rates of NMR-active
nuclei,in particular 13C, because the gyromagnetic ratio
of 2H is 6.5 times smaller than 1H
It improves the resolution and sensitivity of NMR experiments
Which is the ideal level of
deuteration?
It depends from the size of the protein
In general
for c up to 12 ns (20 KDa) 13C/15N labeling
for c up to 18 ns (35 KDa) 13C/15N labeling and fractional
deuteration
for c above 18 ns 13C/15N labeling – selective protonation and
background deuteration
It depends from the type of NMR experiments
The problem to express a
deuterated protein
Incorporation of 2H reduces growth rate
of organisms (up to 50%) and decreases
protein production as a consequence of the
isotopic effect.
Changing a hydrogen atom to deuterium represents a 100% increase in
mass, whereas in replacing carbon-12 with carbon-13, the mass increases
by only 8%. The rate of a reaction involving a C–H bond is typically 6–10
times faster than the corresponding C–D bond, whereas a 12C reaction is
only ~1.04 times faster than the corresponding 13C reaction
Deuterium labeling requires conditions different
with respect to 13C and 15N enrichment and could
require bacteria adaptation
Fractional deuteration
Random fractional deuteration can be obtained up to a
level of 70-75%, in a media with 85% D2O with
protonated glucose, without bacteria adaptation
O/N culture
unlabeled
Preinduction culture
labeled
2-6 hours
OD600=0.3-1.2
Expressing culture
labeled
>20 h
As for 13C, 15N, 2H labelling all the conditions (strain, glucose conc.
time of induction, etc.) must be optimized for each protein!!
Deuterium incorporation
Fractional deuteration of recombinant proteins determined using
mass spectroscopy. ( ) deuteration with [2H]2O only. ( ) deuteration
with [2H]2O and perdeuterated glucose.
O’Connell et al. Anal.Biochem. 1998, 265, 351-355
Perdeuteration
Perdeuteration can require a gradual adaptation of
bacteria to increasing concentration of D2O.
Bacterial strains must be accurately selected in order
to choose that which better acclimates to D2O media.
For each strain one or more colony must be selected
which better survives in high level of D2O concetration
A protocol for bacteria adaptation to
deuterated medium
40% D2O
O/N Inoculum
in unlabelled
medium
60% D2O
80% D2O
99 % D2O
Massive
culture 99 %
D2O
Glycerol stock
40% D2O
Glycerol stock
60% D2O
Glycerol stock
80% D2O
Glycerol stock
99% D2O
Is it possible to avoid the
adaptation phase?
Wüthrich lab has experimented a culture minimal medium
supplemented with deuterated algal hydrolysate which allows us to
eliminate cells pre-conditioning.
Composition of the Celtone-supplemented media
Basic minimal medium
800 ml H2O or D2O
100 ml M9 solution
2 ml 1M MgSO4
1 g
NH4Cl
1 g D-glucose
Vitamin mix and trace elements
10 ml of Vitamin mix
2 ml Trace elements solution
M9 solution
70 g Na2HPO4•7H2O
30 g KH2PO4
5 g NaCl
For a 10X solution, dissolve the ingredients in 1 L of H2O.
Sterilize the solution by autoclaving and dilute it to 1X with H2O
prior to use.
Aminoacids supplements
1-3 g deuterate algal lysate (CELTONE)
dissolved at 30 mg/ml
antibiotics
Wüthrich K. et al J.Biomol.NMR 2004,29,
Is it possible to avoid the
adaptation phase?
SOME RESULTS
Medium composition
Minimal medium on
Glucose + Celtone-d
in H2O
Minimal medium on
Glucose + Celtone-d
in D2O
Deuteration
60-92%
95-97%
Wüthrich K. et al J.Biomol.NMR 2004,29,
Advantage/disadvantages
no N-H/N-D exchange problems
intermediate deuteration can be achieved
high deuteration
Backbone HN
Side-chians
Specific labeling
Labeling of a protein can be easily
achieved on specific residues with 2
strategies:
In
a medium containing small amounts of glucose (13C labelled or
unlabelled)/NH4Cl (15N labelled or unlabelled) and complemented with
the labelled aminoacid(s). A mixture of the other unlabeled
aminoacid(s) can be added to prevent any conversion of the labeled
aminoacid(s)
In a complete labelled medium, containing great amount of all
unlabeled aminoacids except those which are expected to be
labeled.
Specific labeling: the main
problem
The most important problem encountered is
the metabolic conversions of the labeled
aminoacids which might occur during anabolism
and/or catabolism.
How to prevent this?
Use an auxotrophic strain.
Use a prototrophic strain with high concentration of aminoacids
to inhibit some metabolic pathways.
An example: Labeling of a protein with 13C15N Lys can be performed
in unlabeled media with high level of 13C15N Lys to prevent lysine
biosinthesis from aspartate conversion.
Amino Acid Specific Labelling
The protein is produced by expression from bacteria which are grown on minimal
medium supplemented with small amounts of 15NH4Cl and 13C-labelled glucose as well
as labelled and unlabelled amino acids.
The idea is that only those amino acids which are added in labelled form become
labelled in the protein.
Unfortunately, this may not always work as desired, since the E. coli metabolism and
catabolism causes a degree of interconversion between amino acids.
Thus, it is not possible to create a sample with any combination of labelled amino acids.
The situation can be improved somewhat by using auxotrophic bacterial strains
or incorporating enzyme inhibitors.
A cheaper way of labelling only certain amino acids, often called reverse labelling,
involves expression from bacteria which are grown on minimal medium supplemented
with 15NH4Cl and 13C-labelled glucose as well as unlabelled amino acids. This supresses
the labelling of these amino acids and only those which have not been added unlabelled
will be synthesised by the bacteria using the 13C-glucose as the carbon source. Again, a
certain amount of scrambling may occur.
However, if complete control over the incorporation of amino acids is required,
then cell-free methods must be used.
Specific labeling for assignment of
13C
and 1H methyl from Ile, Leu, Val
Full deuteration precludes the use of NOEs
for structure determination.
How to overcome the problem?
Reintroduction of
protons by using
labeled amino acids
Reintroduction of protons
by using methyl selectivelly
protonated metabolic precursors
of aliphatic amino acids or the
biosyntetic precursor of the
aromatic rings.
SAIL - Stereo-Array Isotope Labelling
The basic strategy of the SAIL approach is to prepare amino acids
with the following features:
Stereo-selective replacement of one
1H in methylene groups by 2H.
Replacement of two 1H in each methyl
group by 2H.
Stereo-selective modification of the
prochiral methyl groups of Leu and Val
such that one methyl is 12C(2H)3 and the
other is 13C1H(2H)2.
Labelling of six-membered aromatic rings
by alternating 12C-2H and 13C-1H moieties
The 20 protein-component SAIL amino acids are prepared based on these design
concepts by chemical and enzymatic syntheses.
SAIL - Stereo-Array Isotope Labelling
The production of SAIL proteins involves cell-free expression system. This
approach indeed minimize metabolic scrambing effects and produces high
incorporation rate of the added SAIL amino acid into the target protein.
Specific protonation at ring carbons of Phe,
Tyr, and Trp on deuterated proteins
NOEs involving aromatic protons are an important
source of distance restraints in the structure calculation
of perdeuterated proteins.
A selective reverse labeling of Phe, Tyr and Trp
has been performed in perdeuterated proteins,
using shikimic acid, a precursor of the aromatic
rings.
In this way the aromatic rings of the aminoacids
are partially protonated (50%)
Rajesh S. et al. J.Biomol.NMR 2003, 27, 81-86
Specific protonation at ring carbons of Phe,
Tyr, and Trp on deuterated proteins
Specific protonation at ring carbons of Phe,
Tyr, and Trp on deuterated proteins
The aromatic rings of the aminoacids are partially protonated
(40-56%).
Higher level of protonation are observed in E.coli strains
overexpressing a membrane bound transporter of shikimate
Complete protonation can be achieved using an
auxotrophic strain defective in shikimate production
An example of Site-specific labelling
To obtain CH3 in perdeuterated protein sample:
α-Ketoacid Precursors for Biosynthetic Labeling of Methyl Sites
[1H,13C]-labeled pyruvate as the main carbon source in D2Obased minimal-media expression of proteins results in high
levels of proton incorporation in methyl positions of Ala, Ile(γ2
only), Leu, and Val in an otherwise highly deuterated protein.
A bacterial protein expression system with 13C,1H pyruvate as the sole carbon
source in D2O media
Unfortunately, because the protons of the methyl
group of pyruvate exchange with solvent, proteins are
produced with all four of the possible methyl
isotopomers (13CH3, 13CH2D, 13CHD2, and 13CD3).
IVL - Ile, Val and Leu side-chain methyl groups
The IVL labelling scheme produces protein which is uniformly 2H,13C,15N-labelled,
except for the Ile, Val and Leu side-chains which are labelled as follows:
The protein is produced by expression from bacteria which are grown on
minimal medium in D2O using 13C,2H-glucose as the main carbon source and
15NH Cl as the nitrogen source. One hour prior to induction α-ketobutyrate and
4
α-keto-isovalerate (labelled as shown below) are added to the growth medium
and lead to the desired labelling of the Ile and the Val and Leu residues,
respectively.
Use of α-ketobutyric and α-ketoisovaleric acids as biosynthetic
precursors for the production of deuterated proteins with protonation
restricted to the Ileδ1 and Leuδ/Valγ positions, respectively.
SEGMENTAL LABELLING
Protein splicing is a posttranslational process
in which internal segments (inteins) catalyze
their own excision from the precursor proteins
with consequent formation of a native peptide
bond between two flanking external regions
(exteins). Up to now more than three hundred
inteins have been identified (see
www.neb.com/neb/inteins.html) and many of
them were extensively characterized . Their
self-splicing properties were used to develop
very convenient tools for protein engineering.
There are two methods based on intein
properties that have been used for segmental
isotope labeling of proteins: Expressed Protein
Ligation (EPL) and
Protein Trans-Splicing (PTS).
METODI DI ARRICCHIMENTO
ISOTOPICO
Uniform labeling
All atoms of a selected element are represented by a single isotope
Partial labeling
A selected element is present in a mixture of isotopic forms.
It's not possible to use 15N of the amino acid to label because cell
in which we express the protein have transaminase that make fast
exchange of the label. Deuterium labeling could be done
only for a portion of all hydrogens.
Site-specific labelling
In the site-specific labeling approach only certain residues,
or particular atoms in some residues are isotopically labeled
Minimal media
Trace Elements
In 800 ml H2O dissolve 5 g Na2EDTA and correct to pH 7
Add the following in order, correcting to pH 7 after each:
FeCl3 (.6H20) 0.5 g (0.83 g)
ZnCl2 0.05 g
CuCl2 0.01 g
CoCl2.6H2O 0.01 g
H3BO3 0.01 g
MnCl2.6H2O (.4H20) 1.6 g (1.35 g)
Make up to 1 litre, autoclave and store at 4°C.
M9-minimal media:
Per litre, adds:
7 g Na2HPO4
3 g KH2PO4
0.5 g NaCl
M9-Solution
Then add:
1 ml 1 M MgSO4
200 µl 1 M CaCl2
1 ml Thiamine (40 mg ml-1 stock)
10 ml Trace Elements
Also add, as necessary:
15 ml Glucose (20 % Stock) (gives 0.3 % final)
1 g NH4Cl