Recombination - the Department of Statistics
advertisement

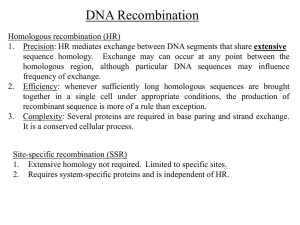
Recombination, and haplotype structure Simon Myers, Gil McVean Department of Statistics, Oxford The starting point • We have a genome’s worth of data on genetic variation • We wish to understand why the haplotype structure looks how it does – Differences between regions, populations Where do haplotypes come from? • In the absence of recombination, the most natural way to think about haplotypes is in terms of the genealogical tree representing the history of the chromosomes • • Tree affects mutation patterns Mutation patterns give information on tree What determines the shape of the tree? Present day Ancestry of current population Present day Ancestry of sample Present day The coalescent: a model of genealogies Most recent common ancestor (MRCA) coalescence Ancestral lineages Present day time Simulating histories with the coalescent Simulating data with the coalescent Haplotype structure in the absence of recombination • In the absence of recombination, the shape of the tree and where mutations fall on it determine patterns of haplotype structure • Two mutations on the same branch will be in complete association, mutations on different branches will have lower and often low association r2 = 1 r2 = 0.04 Haplotypes when there is recombination • When there is no recombination, haplotype structure reflects the age distribution of mutations and the shape of the underlying tree • When there is some recombination, every nucleotide position has a tree, but the tree changes along the chromosome at a rate determined by the local recombination landscape • By using SNP information to inform us about the trees, we can learn about how quickly the trees changes – This relates to the recombination rate A bit of recombination ‘shuffles’ genetic variation Lots of recombination does lots of shuffling Recombination and haplotype diversity • Without recombination, a new mutation can create at most one new haplotype – Any two mutations delineate at most 3 haplotypes in total (ancestral, plus two new types) • With recombination, this mutation can spread onto every existing haplotype background, creating the potential for more haplotypes • For a given number of SNPs a region with recombination will tend to have (in comparison to a region with no recombination) – More haplotypes – Less variance in the pairwise differences between haplotypes – Less skewed haplotype frequencies The ancestral recombination graph • The combined history of recombination, mutation and coalescence is described by the ancestral recombination graph Event Coalescence Mutation Coalescence Coalescence Mutation Coalescence Recombination In humans, recombination is not uniformly distributed • Most recombination occurs in recombination hotspots – short (1-2kb) regions every 50-100kb that occupy at most 3% of the genome but probably account for 90% or more of the recombination • This means that haplotype structure in humans is an interesting hybrid between the no recombination and lots of recombination situations Learning about recombination • Just like there is a true genealogy underlying a sample of sequences without recombination, there is a true ARG underlying samples of sequences with recombination • We can consider nonparametric and parametric ways of learning about recombination • There are useful nonparametric ways of learning about recombination which we will consider first – These really only apply to species, such as humans, where we can be fairly sure that most SNPs are the result of a single ancestral mutation event The signal of recombination? Ancestral chromosome recombines Recurrent mutation Recombination Detecting recombination from DNA sequence data • Look for all pairs of “incompatible” sites • Find minimum number of intervals in which recombination events must have occured (Hudson and Kaplan 1985): Rm Improving the detection algorithm • Rm greatly underestimates the amount of recombination in the history of a set of sequences • Myers and Griffiths (2003) developed an improved way of detecting recombination events – Without recombination, every new mutation can create only a single new haplotype – With recombination, mutations can be shuffled between haplotype background, generating haplotype diversity – Each recombination makes at most one new haplotype – If I see H haplotypes with S segregating sites, at least H-S-1 recombination events must have occurred • This offers potential to identify many more recombination events – Carefully combine bounds from different collection of sites – Dynamic programming algorithm makes computation extremely fast – Better (sometimes slower) algorithms developed recently Problems with ‘counting’ recombination events A tree-pair where we could see recombination events, but don’t Tree-pairs where we cannot see recombination events Modelling recombination • Model-based approaches to learning about recombination allow us to ask more detailed questions than nonparametric approaches – What is the rate of recombination (as opposed to just the number of events) – Does gene A have a higher recombination rate than gene B? – Is the rate of recombination across a region constant? – Where are the recombination hotspots? • We can use coalescent model approaches (approximations) to calculating the likelihood of arbitrary recombination maps given observed data Fitting a variable recombination rate • Cold Hot Use a reversible-jump MCMC approach (Green 1995) SNP positions Split blocks Merge blocks Change block size Change block rate Acceptance rates ( , ) m in 1, Composite likelihood ratio ( , u ) ( ) ( ) q ( , ) ( , u ) C C ( ) ( ) q ( , ) Ratio of priors Hastings ratio Jacobian of partial derivatives relating changes in dimension to sampled random numbers • Include a prior on the number of change points that encourages smoothing Strong concordance between fine-scale rate estimates from sperm and genetic variation Rates estimated from genetic variation McVean et al (2004) Rates estimated from sperm Jeffreys et al (2001) Inferring hotspots • We perform a statistical test for hotspot presence • Based on an approximation to the coalescent similar to that used for rate estimation • All previously identified hotspots are 1-2kb in size – – – – – – At a position in genome, consider where 2kb hotspot might be present Fit a model with hotspot Fit one without Compare in terms of (approximate) likelihood ratio test Evaluate significance via simulation When p-value below threshold, declare a hotspot Rates and hotspots across the human genome Hotspots throughout human genome (35,000 identified) From Myers et al. (2005) Applications of recombination approaches to real data • Rates and hotspots across the human genome (Myers et al. 2005) – Previously, no understanding of why hotspots localise where they do – Can 35,000 hotspots, accounting for >50% of human recombination, help? • Comparison of recombination rates (Winckler et al. 2004, Ptak et al. 2005) – Between humans and chimpanzees – At individual recombination hotspots • Understanding genomic rearrangements (Myers et al., submitted!) – Cause a number of “genomic disorders” – Relationship to recombination hotspots 32,996 Phase II HapMap hotspots Estimated 50-70% of all human recombination Hotspots on all chromosomes, including X THE1B (LTR of retrotransposon) ~20,000 hotspots localised to within 5kb THE1B: Found in 1196 hotspots versus 606 coldspots (p<<10-20) AluY: Found in 3635 hotspots versus 3262 coldspots (p=7x10-5) THE1 consensus: ...CTTCCGCCATGATTGTGAGGCCTCCCCAGCCATGTGGAACTGTGAGTCCATT... CCTCCCTAGCCAC (n=165) CCNCCNTNNCCNC (n=263) CCTCCCCNNCCAT (n=10,690) ~3-4% of hotspots L2 consensus: ...TGTCACCTCCTCAGAGAGGCCTTCCCTGACCACCCTATCTAAAATWGCACACC... CCTCCCTGACCAC (n=157) CCNCCNTNNCCNC (n=6,901) CTTCCCTNNCCAC (n=1,211) ~3-4% of hotspots AluY, AluSc, AluSg consensus: ...CTCCTGACCTCGTGATCCGCCCGCCTCGGCCTCCCAAAGTGCTGGGATTACAG... CCGCCTTGGCCTC (n=14,028) CCNCCNTNNCCNC (n=15,706) CCGCCTCNNCCTC (n=55,916) ~3-4% of hotspots, including DNA3 Human hotspot motifs • In humans, specific words produce recombination hotspot activity • Hotspot motif CCTCCCTNNCCAC (p<10-33) – – – – – – Raises probability of a hotspot across genetic backgrounds Degenerate versions CCNCCNTNNCCNC and truncated CCTCCCT also raise probability, to lesser extent Motif explains ~40% of human hotspots Operates in both sexes We don’t know, very clearly, which hotspots On THE1 background, hotspot 70-80% of time! • Biology not clearly understood • We identified a second, different hotspot motif (the best 9bp motif), CCCCACCCC, also by comparison of hot and cold regions of the genome Variation in individual hotspots Sequence variation affects recombination at DNA2 (Jeffreys and Neumann, Nature Genetics 2002) SNPs disrupting hotspots disrupt motifs! • DNA2: Jeffreys and Neumann (Nature Genetics 2002, Hum Mol. Evol. 2005) • Hot AAAAGACAGCCTCCCTGTTGCTGC Cold AAAAGACAGCCCCCCTGTTGCTGC NID1: Hot Cold CACCCCCCACCCCACCCCAACATA CACCTCCCACCCCACCCCAACATA SNPs disrupting hotspots disrupt motifs • DNA2: Jeffreys and Neumann (Nature Genetics 2002, Hum Mol. Evol. 2005) Hot AAAAGACAGCCTCCCTGTTGCTGC Cold AAAAGACAGCCCCCCTGTTGCTGC Disruption of CCTCCCT, best 7bp motif • NID1: Hot Cold CACCCCCCACCCCACCCCAACATA CACCTCCCACCCCACCCCAACATA Disruption of CCCCACCCC, best 9bp motif Role of motif in X-linked ichthyosis VCX2 1/5000 births Deletion breakpoint hotspot (Van Esch et al. 2005) • The 1kb deletion hotspot contains 25 repeats of CCTCCCTNNCCAC • Highest motif density in any LCR in entire genome • Strongly implicates motif in producing hotspot • Points to a link between deletion-causing and “normal” recombination A more general link? • Many other diseases are caused by recombination-mediated deletions and duplications (NAHR) – – – – • Smith-Magenis syndrome (hotspot) CMT1A (hotspot) NF1 microdeletion syndrome (hotspot) DiGeorge syndrome…. Two recent studies suggest normal hotspots and hotspots of disease-causing deletion may coincide – – de Raedt, Stephens et al. (Nature Genetics, 2006) Two NF1 deletion hotspots both likely to coincide with crossover hotspots – – Lindsay et al. (ASHG, 2006) CMT1A deletion hotspot associated with crossover hotspot Other “major” NAHR hotspots CCNCCNTNNCCNC overrepresented in hotspots p=0.0006 Evolution of recombination – human vs. chimps LDhot hotspots Human Chimp LDhat rate estimates No significant correlation in hotspots positions between species (Winckler et al. Science 2005, Ptak et al. Nature Genetics 2005) Reading • Haplotype structure and recombination – The International HapMap Consortium: A haplotype map of the human genome. Nature 2005, 437:1299-1320. – McVean G, Spencer CCA, Chaix R: Perspectives on human genetic variation from the International HapMap Project. PLoS Genetics 2005, 1:e54. – Myers S, Bottolo L, Freeman C, McVean G, Donnelly P: A fine-scale map of recombination rates and recombination hotspots in the human genome. Science 2005, 310:321–-324. • The coalescent – Nordborg M: Coalescent Theory. In The Handbook of Statistical Genetics (eds Balding, Bishop and Cannings), 2001. Wiley & Sons. – Hudson RR: Gene genealogies and the coalescent process. In Oxford Surveys in Evolutionary Biology (eds Futuyama and Antonovics) 1990, 7:1–44. Oxford University Press. Selected references - Jeffreys, A.J., L. Kauppi, and R. Neumann. 2001. Intensely punctate meiotic recombination in the class II region of the major histocompatibility complex. Nat Genet 29: 217-222. - Jeffreys, A.J. and R. Neumann. 2002. Reciprocal crossover asymmetry and meiotic drive in a human recombination hot spot. Nat Genet 31: 267-271. - Jeffreys, A.J. and R. Neumann. 2005. Factors influencing recombination frequency and distribution in a human meiotic crossover hotspot. Hum Mol Genet 14: 2277-2287. - Myers, S., L. Bottolo, C. Freeman, G. McVean, and P. Donnelly. 2005. A fine-scale map of recombination rates and hotspots across the human genome. Science 310: 321-324. - Ptak, S.E., D.A. Hinds, K. Koehler, B. Nickel, N. Patil, D.G. Ballinger, M. Przeworski, K.A. Frazer, and S. Paabo. 2005. Fine-scale recombination patterns differ between chimpanzees and humans. Nat Genet 37: 429-434. - The International HapMap Consortium. 2005. A haplotype map of the human genome. Nature 437: 1299-1320. - The International HapMap Consortium. 2007. The Phase II HapMap. Nature - Winckler, W., S.R. Myers, D.J. Richter, R.C. Onofrio, G.J. McDonald, R.E. Bontrop, G.A. McVean, S.B. Gabriel, D. Reich, P. Donnelly et al. 2005. Comparison of fine-scale recombination rates in humans and chimpanzees. Science 308: 107-111.