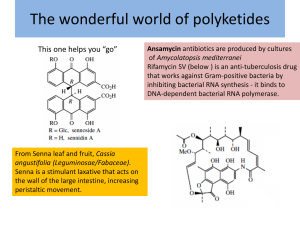
1. Introduction to Natural Products Chemistry
advertisement

5. Polyketides RA Macahig FM Dayrit Introduction • Polyketides (which literally means “many ketone groups”) make up a diverse biogenetic group which starts from acetylCoA to form a linear chain without extensive reduction. The polyketide chain can cyclize to form aromatic rings or undergo extensive derivatization. • Polyketides rank among the largest group of secondary metabolites in terms of diversity of structure and biological diversity. • Polyketide biosynthesis shares some similarities with the initial steps of fatty acid acetyl polymerization. Like the fats, the polyketide pathway probably arose early in biological evolution before the rise of plants. 5. Polyketides (Dayrit) 2 Examples of polyketide natural products which illustrate the wide variety of structures which comprise this group. OH CH3 O OCH3 CO2H HO OCH3 HO OH O O OH orsellinic acid O H3C CH3O Cl O griseofulvin from Penicillium griseofulvum alternariol from the mould Alternaria tenius O O H3C CH3 HO O O O OH H3C CH3 O O OCH3 aflatoxin B1 from Apergillus species macrolide antibiotic erythromycin-type from Streptomyces species OR CH3 O OR CH3 5. Polyketides (Dayrit) 3 Introduction • The polyketides have great diversity of structures and chemical functionalities. These structures range from saturated macrocyclic lactones (macrolides), which are unique polyketide metabolites, to various types of aromatic compounds. • Polyketides occur widely in bacteria, fungi and lichens, but are of relatively minor occurrence in higher plants. Bacteria, in particular Actinomycetes and Cyanobacteria, are prolific sources of polyketides, many of which possess antibiotic activity. Other significant polyketide producers are Aspergillus (aflatoxins) and Penicillium and Streptomyces species (tetracycline antibiotics). 5. Polyketides (Dayrit) 4 Overview of polyketide biosynthesis Polyketides are produced from poly-acetyl intermediates (poly-1,3-diketo compounds) which do not undergo complete reduction, as in the case of the fats. The polyketides then branch into two major pathways: 1. Aromatic compounds. The reactive 1,3-diketo groups undergo intramolecular Claisen or lactonization reactions forming cyclic compounds. Dehydration produces aromatic compounds. 2. Macrolides. The keto- groups are reduced to alcohols, which are subsequently dehydrated to form linear compounds. The final products are macrocyclic esters. Macrolides generally >12 carbon atoms in the ring. 5. Polyketides (Dayrit) 5 Aromatic polyketides. Major cyclization pathways for a tetraketide followed by aromatization. O c c d d O O O O 7 8 C 6 5C 4 3C 2 C1 H3C CH2 CH2 CH2 S-CoA a b a b a b d c O S-CoA O O O S-CoA O O O O S-CoA O O O O Claisen 6 1 O Aldol 2 7 (8) CH3 O C O (8) HO S-CoA O O (1) OH CH3 (1) HO (1) O CO2H O OH xanthoxylin (a phloroglucinol) HO OH orsellinic acid (a resorcinol) 5. Polyketides (Dayrit) O CH2 (a -pyrone) CH2 C (8) H3C O (8) CH3 (an -pyrone) 6 (1) CO2H Biosynthetic studies on polyketides (Arthur Birch) 4x ratio: H3C O* O* O* O* O* _ C # O* # # # # S-CoA # 1 ------ = ---- 14 C # 1 --------- = ---18 O * HO * * 2 CH3 O # # # # * OH 1 * OH *OH O* -H 2O* # # *O # S-CoA # O* orsellinic acid The elucidation of the polyketide pathway was pioneered by Arthur Birch in 1953. Birch used 14C and 18O-labeled acetate which he fed to microorganisms to establish the incorporation pattern and from this to postulate the steps in the biosynthesis of polyketides. # 4 ------ = ---* 3 5. Polyketides (Dayrit) 7 Overview of polyketide biosynthesis Birch proposal for polyketide biosynthesis: 1. Starting with a starter unit, C2 units are added to form the polyketide chain (chain assembly). 2. Reduction and/or alkylation of the polyketide chain before cyclization. 3. Intra- or intermolecular cyclization. (The more common pathway is intramolecular cyclization.) 4. Secondary processes which modify the intermediate product after cyclization, such as: halogenation, Omethylation, C-methylation, reduction, oxidation, decarboxylation and skeletal rearrangement. 5. Polyketides (Dayrit) 8 CH3 O Short-hand representation of polyketides: o O a tetraketide S-CoA O o = o o O Variations in number of C2 units and mode of cyclization Starting polyketide Secondary metabolite HO triketide: o o o O O CH3 tetraketides: (1) CO2H o o o orsellinic acid o HO OH OH o O o CH3 o xanthoxylin (1) o HO OH 5. Polyketides (Dayrit) 9 Variations in number of C2 units and mode of cyclization Starting polyketide Secondary metabolite pentaketides: HO o o CH3 o O (1) o o OH O OH o O o curvulinic acid (Curvularia siddiqui) CH3 o o o CO2H HO (1) OH o o o O o o HO (1) O CH3 hexaketide: o o o o CH3O CH3 O o diaporthin (Endothia parasitica) OH o OH O 5. Polyketides (Dayrit) 10 Variations in number of C2 units and mode of cyclization Starting polyketide Secondary metabolite heptaketide: O o o o o o CH3O O o o monocerin (Helminthosporium monoceras) CH3O OH OCH3 o o o o O O o OCH3 O o o O H3C CH3O griseofulvin (Penicillium griseofulvum) Cl HO CH3 o o o o OH alternariol (Alternaria tenius) o o o HO O O 5. Polyketides (Dayrit) 11 Variations in number of C2 units and mode of cyclization Starting polyketide Secondary metabolite octaketide: O HO o o o o o CH3 o o CO2H o OH O O o o o curvularin (Curvularia spp.)) o o o HO nonaketide: HO o o o O O CH3 o o o o o HO O Cl o o o o o o o O radiciciol (Nectaria radiciola) O o O HO o o endocrocin (Centralia endocrocea) o O CH3O CH3 OH o 5. Polyketides (Dayrit) HO O OH nalgiovensin (Penicillium nalgiovensis) 12 O A. Example of intermolecular cyclization. Inter- vs. intramolecular cyclization: A. Colletodiol; B. Use of labeling experiments to distinguish intra- from intermolecular cyclization. o o o o o o O O O o OH OH colletodiol B. Use of labeling experiment to distinguish inter- vs. intramolecular cyclization. [Me*] o o o o o o o o o o -CO2 o o * o o 5. Polyketides (Dayrit) -2CO 2 OH OH O o 13 Biosynthesis of macrolides: Step-wise chemical transformations and enzymes. (from: The World of Polyketides, http://linux1.nii.res.in/) 5. Polyketides (Dayrit) 14 * O O O HS HS HS HS HS S-CoA H3C HS HS HS HS S _ O 2C 4 x S -Co A multi-enzyme complex Hypothetical scheme of the biosynthesis of phenol polyketides on the Polyketide Synthase (PKS) multienzyme complex. _ _ O 2C _ O 2C O 2C o _ * _ O 2C O 2C o O O O O O S S _ O 2C S S S S HS S S * S O O _ O O _ base O O * O O S HS HS HS O HS CO2H S * HO O O OH O O OH 5. Polyketides (Dayrit) * O O O O _ O 2C O * 15 S Polyketide synthase (PKS) The PKS family share a number of characteristics with the family of fatty acid synthases (FAS): the PKS is a multienzyme complex which is arranged so that the stepwise transformations are carried out sequentially. CO2H H3C OH 6-Methy lsalicy lic acid O OH O O (from: The World of Polyketides, http://linux1.nii.res.in/) O CH3 CH3 H3C Lov astatin • ACP: acyl carrying protein • KS: b-keto acyl synthase • MAT: malonyl (acyl) transferase • DH: dehydratase • ER: enoyl reductase • KR: keto reductase • TE: thiol esterase Hypothetical model for one type of PKS multienzyme system which produces 6-methylsalicylic acid and lovastatin. The growing chain is assembled on two multienzyme complexes. 5. Polyketides (Dayrit) 16 The biosynthetic pathway for the fungal polyketide 6-methylsalicylic acid (6-MSA). 6-MSA is assembled KS: ketosynthase MAT: malonyl-acetyl transferase DH: dehydratase KR: ketoreductase ACP: acyl carrier protein from four ketide units (one acetate and three malonates). 6-MSAS contains the following domains (in order): KS, MAT, DH, KR and ACP. These act repeatedly to catalyse three rounds of chain extension, carrying out different levels of reductive processing at each stage. The first condensation is followed by reaction with a second equivalent of malonate extender unit, while the second condensation is followed by reduction and dehydration of the newly-formed keto group. After the third cycle, the chain undergoes cyclisation, dehydration and enolisation. The absence of a thioesterase domain suggests that release of the chain from the PKS does not occur by hydrolysis but by an alternative mechanism which is still not verified. (Staunton and Weismann, Nat. Prod. Rep., 2001, 18, 380–416) Biosynthesis of macrolides on a modular Polyketide Synthase (PKS) multienzyme complex. (from: The World of Polyketides, http://linux1.nii.res.in/) 5. Polyketides (Dayrit) 18 Domain organization of the erythromycin polyketide synthase. Putative domains are represented as circles. Each module incorporates the essential KS, AT and ACP domains, while all but one include optional reductive activities (KR, DH, ER). The one-to-one correspondence between domains and biosynthetic transformations explains how programming is achieved in this modular PKS. (Staunton and Weismann, Nat. Prod. Rep., 2001, 18, 380– 416) 5. Polyketides (Dayrit) 19 KS: ketosynthase AT: acyltransferase DH: dehydratase ER: enoyl reductase KR: ketoreductase ACP: acyl carrier protein TE: thioesterase Predicted domain organization of the 6-deoxyerythronolide B synthase (DEBS) proteins. KR indicates the inactive ketoreductase domain. The ruler shows the residue number within the primary structure of the constituent proteins. The linker regions are also given in proportion. (Staunton and Weismann, Nat. Prod. Rep., 2001, 18, 380–416) 5. Polyketides (Dayrit) 20 KS: ketosynthase AT: acyltransferase DH: dehydratase ER: enoyl reductase KR: ketoreductase ACP: acyl carrier protein TE: thioesterase Inactivation of KR5 of DEBS results in the production of erythromycin analogues with keto groups at the C-5 position. (Staunton and Weismann, Nat. Prod. Rep., 2001, 18, 380–416) 5. Polyketides (Dayrit) 21 KS: ketosynthase ER: enoyl reductase TE: thioesterase AT: acyltransferase KR: ketoreductase DH: dehydratase ACP: acyl carrier protein Inactivation of ER4 results in an analogue of erythromycin with a double bond at the expected site. (Staunton and Weismann, Nat. Prod. Rep., 2001, 18, 380–416) 5. Polyketides (Dayrit) 22 Domain organization of the rapamycin polyketide synthase (RAPS). As with the erythromycin PKS there is a co-linearity between the sequence of modules and the order of biosynthetic steps. (Staunton and Weismann, Nat. Prod. Rep., 2001, 18, 380–416) 5. Polyketides (Dayrit) 23 What is the link between FAS and PKS? The PKS system is likely derived from bacterial FAS. Different PKS pathways in bacteria illustrate the selective evolutionary advantage that multiple secondary metabolite biosyntheses confer to individual bacteria and taxonomic kingdoms. KS: ketoacyl synthase AT: acyl transferase DH: dehydratase ER: enoyl reductase ACP: acyl carrying protein Organization of fatty acid synthases (FAS) and polyketide synthases (PKS). (Jenke-Kodama et al. J Mol Bio Evol 2005) 5. Polyketides (Dayrit) 24 What is the link between FAS and PKS? Common sequence of reactions performed by FAS and PKS. Enzymes in a PKS module. (Jenke-Kodama et al. J Mol Bio Evol 2005) KS: ketoacyl synthase ACP: acyl carrying protein KR: ketoreductase DH: dehydratase ER: enoyl reductase 5. Polyketides (Dayrit) 25 Common enzymes in aromatic polyketides O O - O2C S-CoA min PKS starter unit R O O O O O O KS-CLF O O O O SACP R 15 O 9 O O O SACP OH C-9 KR OH O OH R O O CYC O S-ACP R O O 5 O O O ARO O SACP HO R O O 9 OH O O O SACP principal common intermediate with varying R group common aromatic intermediate Four proteins comprise the minimal PKS: ketosynthase (KS), chain length factor (CLF), acyl carrier protein (ACP), and a malonyl-CoA:ACP transacylase (MAT) which is usually recruited from fatty acid synthases. Other common enzymes include: aromatase (ARO) and cyclase (CYC). (Ridley et al., PNAS, 2008, 105:4595-4600) 5. Polyketides (Dayrit) 26 “Deciphering the mechanism for the assembly of aromatic polyketides by a bacterial polyketide synthase,” Shen and Hutchinson, Proc. Natl. Acad. Sci. USA, 93, 6600-6604, June 1996. aberrant cyclization Acy l-CoA + 9 Mal-CoA unidentif ied products O O TcmJKLM O O O O O O HO SCoA O OH TcmN O CH3 O OH 0 Tetracenomy cin PKS 7 kb K J M L OH OH CO2H CH3 Tcm F2 N TcmI O O CH3O OH HO TcmN O OH Tcm B3 CH3 OH TcmH CO2H OH HO OH CO2H CO2H OH O OH CH3 Tcm D3 OH O OH CH3 Tcm F1 The optimal Tcm PKS is a complex consisting of the TcmJKLMN proteins. It is the integrity of this complex that maximizes the efficiency for the synthesis of aromatic polyketides from acetyl- and malonyl-CoA. 5. Polyketides (Dayrit) 27 Polyketide modifications: before cyclization and after cyclization (secondary processes). Note: F: fungi; P: plant refers to the biological system where the process has been studied. The number of marks denote frequency of occurrence; denotes not observed. Modification 1. reduction 2. oxidation 3. C-methyation 4. O-methylation 5. C-prenylation 6. O-prenylation 7. C-glycosylation 8. O-glycosylation 9. decarboxylation 10. aromatic radical coupling Before cyclization (F) (F) (F,P) After cyclization (Secondary process) ? (F,P) (F,P) (F,P) (P) (P) (P) • The various Kingdoms exhibit different characteristics of their PKS enzymes. In the microbial kingdom, at least three types of PKS enzymes have been recognized. 5. Polyketides (Dayrit) 28 Reduction and alkylation of the polyketide chain before cyclization. The polyketide can be reduced to the alcohol and be subsequently dehydrated to produce the double bond. The resulting aromatic ring will not have a OH substituent in the particular position. O O O + S-CoA _ 2 x O2C O O S-CoA S-CoA 1. NADPH 2. -H2O O O O _ O2C O S-CoA S-CoA S-CoA O O CH3 o o CO2H 6-methylsalicylic acid from Penicillium urticae o OH 5. Polyketides (Dayrit) 29 Reduction and alkylation of the polyketide chain before cyclization. The polyketide can be C-alkylated (e.g., with methyl or isopentyl groups) prior to cyclization although it may be difficult to determine whether C-alkylation is carried out before or after cyclization. OH O CH3 OH [CH3 ] o o o O HO H3C CH3 OH o HO [CH3 ] H3C CH3 clavatol 5. Polyketides (Dayrit) O CH3 HO 30 Secondary processes: examples of oxidation, decarboxylation and methylation. A. Gentisic acid CH3 CO2H CO2H CHO CHO HO CO2H [O] [O] -CO2 OH OH OH OH gentisic acid 6-methylsalicylic acid B. Fumigatin CH3 CH3 CO2H 1. [O] 2. [CH3] -CO2 HO OH HO CH3 CH3 OH orsellinic acid O HO [O] HO OH OCH3 HO O OCH3 fumigatin 5. Polyketides (Dayrit) 31 Erythromycin, first * isolated from Streptomyces erythreus from soil samples from Iloilo sent by Abelardo Aguilar in 1949. It was first marketed by Eli Lilly as Ilosone®. R.B.Woodward accomplished its stereospecific synthesis in 1981. It is used for the treatment of grampositive bacterial infections. O O + 6 S-CoA S-CoA starter unit 7 o 9 o 11 o o5 CO2H 13 o Erythromycin A B C R1 OH H OH R2 1 1 1 o o 1 R3 2 2 3 3 * CH3 O O 9 1 : D-desosamine: N(CH3)2 OH HO 2 : L-cladinosine: O CH3 CH3 11 HO * 7 OR1 OR2 O 1 3 O OR3 CH3 HO 3 : L-mycarose: O CH3 OH 5. Polyketides (Dayrit) CH 3 OH 5 13 32 5. Polyketides (Dayrit) 33 Intramolecular aromatic radical coupling: biosynthesis of griseofulvin (from a fungus, Penicillium griseofulvum) involves extensive secondary modification of a heptaketide. OH o o O OH o OH o o o o HO OH + 2 [CH3 ] CH3 OH O OCH 3 OH OH CH3 O CH3 O CH3 O OCH 3 OH griseophenone C OH O OCH 3 [O] OH Cl O . CH3 O CH3 Cl O O. CH3 O O CH3 O OCH 3 . . OH CH3 O OH CH3 griseophenone B + [CH3 ] OCH3 OH OH Cl O OCH 3 + [Cl], -[H] CH3 Cl griseophenone A OH O OH O OCH3 OCH3 O CH3 O O CH3 Cl 5. Polyketides (Dayrit) griseofulvin + 2 [H] O CH3 O O CH3 Cl dehydrogriseofulvin 34 Nature of starting unit Fatty acid s ynthas e (FAS) Polyk e tide s ynthas e (PKS) O O O O C C H3C H3C SCoA Acety l CoA SCoA Acety l CoA SCoA OH Propiony l CoA Benzoy l CoA O O C HO O O C CH2 SCoA Malony l CoA O OH Buty ry l CoA SCoA R2 O O C CH R1 Isobuty ry l CoA O C HO O OH SCoA CH3 Methy lmalony l CoA R OH Hexanoy l CoA, R=C 5H11 Octanoy l CoA, R=C 7H15 O H3C SCoA Acetoacety l CoA O R1 R2 Cinnamoy l CoA H p-Coumaroy l CoA H Caf f eoy l CoA OH Feruloy l CoA OH H OH OH OMe O O MeHN H2N SCoA SCoA Acetamidoacety l CoA N-Methy lanthrany loy l CoA 5. Polyketides (Dayrit) 35 Nature of starting unit Examples of metabolites where the starting unit is not acetyl-CoA. In the case of tetracycline, extensive secondary processes take place. o CO2H OH O OH o o o o o O HO o o o o HO OH 7S, 9R, 10R--pyrramycinine Cl H C 3 o o o o o H N(CH3)2 o o o OH o OH CONH2 CONH2 OH OH O OH O tetracycline 5. Polyketides (Dayrit) 36 Metabolites from polyketides The polyketide metabolites can be classified into five groups: 1. Phenols 2. Quinones Aromatic compounds 3. Aflatoxins 4. Tetracyclines 5. Macrolide antibiotics 1. Phenols Cyclization and aromatization of polyketides form phenols as the initial product. In plants however, phenols are also formed from the shikimate pathway. Therefore, phenols and their methylated derivatives are common natural products. Some common phenols are formed via different pathways. 5. Polyketides (Dayrit) 37 Metabolites from polyketides 2. Quinones Quinones often occur as the final product from a series of oxidation reactions on mono- or polycyclic aromatic ring systems. The biosynthetic pathway differs in microorganisms and plants. In microorganisms, quinones arise predominatly via the polyketide pathway. In plants, however, quinones can arise via the polyketide or shikimate pathways and sometimes via the mixed biosynthetic route involving the ring-formation of an added terpenoid unit. The presence of multiple pathways to the quinone ring system may reflect the importance of this type of functionality. 5. Polyketides (Dayrit) 38 In microorganisms: [O] polyketide Overview of biosynthesis of quinones. Depending on the organism, quinones can arise via the polyketide or shikimate pathways. • Aromatic metabolites in microorganisms are likely to be formed via the polyketide pathway while aromatic compounds in plants are likely to come from the shikimate pathway. aromatic compound quinone In plants: [O] polyketide aromatic compound quinone quinone shikimate aromatic compound + terpene [O] quinone (mixed metabolite) quinone from shikimate: OH quinones from shikimate + terpene: OH O OH O H OH CO2H OH homogentisic acid alkarinin O R H O 39 n ubiquinones: R = H, CH3 ; n = 4-13 Metabolites from polyketides 1,4-Benzoquinone 1,4-Benzoquinone itself is the simplest member of this group. However, because it is toxic, it is not found in this form but rather as a protected precursor, such as arbutin, a glycosylated 1,4-hydroquinone, the reduced form of 1,4benzoquinone. O-Glu Arbutin occurs in the leaves of various plant species and may be a plant defense compound. The ability to detoxify phenols or to store them as glycosides appears to be a common characteristic of plants. 5. Polyketides (Dayrit) OH Arbutin 40 5. Polyketides (Dayrit) 41 A. Plants store precursors of para-quinone in various glycosylated forms. O-Glucose Para-quinone is a toxic compound which various organisms use. A. Various trees secrete a precursor (arbutin) to “clear” its surroundings of competing plants; B. The bombardier beetle produces paraquinone in its collecting bladder from parahydroquinone + H2O2. OH arbutin O-Glu-O-Glu O-Glu-O-Glu-O-Glu OH OH O para-quinone (toxic) O B. Para-quinone as a defensive secretion of the bombardier beetle. collecting bladder OH lobe + H2 O2 explosion chamber with enzyme gland OH O + H2 O + heat 5. Polyketides (Dayrit) O 42 5. Polyketides (Dayrit) 43 Metabolites from polyketides Aflatoxins • The aflatoxins are a group of fungal metabolites which have closely similar chemical structures, the most evident feature being two fused furan rings. • Aflatoxins were first discovered following investigations into the deaths of turkeys after being being fed mouldy peanuts. O O O O O OCH3 af latoxin B1 f rom Apergillus species 5. Polyketides (Dayrit) 44 Metabolites from polyketides Aflatoxins • Aflatoxins are among the most toxic naturally-occuring compounds known. They are potent hepatocarcinogens and cause lesions in the mammalian liver. They are toxic to rats down to a dose level of 1 g/day. • Various strains of Aspergillus produce aflatoxins, in particular, A. parasiticus, A. versicolor and A. flavus. Aspergillus fungi are usually encountered growing on various types of organic matter, especially in damp places. They cause the decay of many stored fruits and vegetables, bread, leather goods and various fabrics. • Aflatoxins are one of the major causes of concern in our copra industry. The European Commission limit is currently set at 5 ppb. 5. Polyketides (Dayrit) 45 O [O] HO o o o o o o o o o decaketide Aflatoxins make up a family of polyketide metabolites. The very complex biosynthesis of aflatoxins was elucidated by George Büchi. OH o OH O OH O O +2[H] O +2[H], -H 2O, +2[H] O O HO O HO + H O OH HO OH O OH OH OH O OH OH -H2 O O O HO [O] O HO OH O OH OH O OH O OH OH -H 2O O HO OH CHO CHO O -C2 O HO OH OH OH O HO O O OH O-H CHO O O OH OH O-H O OH O [O] averufin O O HO OH OH O O OH versicolorin B versicolorin A 5. Polyketides (Dayrit) [O], Bayer-Villiger 46 O +2[H] O HO OH O HO [O] Bayer-Villiger O O CO2H HO OH OH O O OH versicolorin A +2[H], -CO2 O H [O] O H O OH OH [O] H O H [O] H O O OH OH sterigmatocystin O O OH O O HO O O O OH OH H O O O H O HO2C O O OH O H O O O OH H H H O O H O O -CO 2 , +[CH 3 ], -H 2 O O O H O O O H O O O O O O OH OCH3 aflatoxin B1 OH 5. Polyketides (Dayrit) CO2H [CH 3 ] _ O CO2H OH 47 O [O] HO o o o o o OH o o o o o decaketide OH O OH O O +2[H] O +2[H], -H 2O, +2[H] O O HO O HO + H O OH HO OH O OH OH OH O OH OH -H2 O O O HO [O] O HO OH O OH OH O OH O OH OH OH O-H [O] averufin -H 2O O O HO O 5. OH -C2 Polyketides (Dayrit) CHO CHO HO O OH 48 O-H OH O OH OH OH O OH OH -H2 O O O HO [O] O HO OH O OH OH O OH O OH OH O-H -H 2O O O HO OH CHO CHO O -C2 O HO OH OH OH O HO O O OH O-H CHO O O OH OH [O] averufin OH O OH OH O O OH versicolorin B versicolorin A 5. Polyketides [O], Bayer-Villiger O HO O (Dayrit) 49 O +2[H] O HO OH O HO [O] Bayer-Villiger O O CO2H HO OH OH O O OH versicolorin A +2[H], -CO2 O H [O] O H O OH OH [O] H O O H O OH O O OH O O HO O O OH OH sterigmatocystin H [O] O H O O 5. PolyketidesOH (Dayrit) O OH H O HO2C O O O H O O O 50 OH O H [O] O H O OH OH [O] H O OH OH H [O] H O O sterigmatocystin O O OH O O HO O O O OH OH H O O O H O HO2C O O OH O H O O O OH H H H O O H O O -CO 2 , +[CH 3 ], -H 2 O O O H O H O O O O O O OH OCH3 aflatoxin B1 5. Polyketides (Dayrit) CO2H O O OH [CH 3 ] _ O CO2H OH 51 +2[H] [O] [CH 3] CH3 OH OH o o o o o NH2 o o o o CONH2 HO CH3 H HO OH OH [O] CH3 O H O O OH OH NH2 NH2 Biosynthesis of tetracyclines from Streptomyces species. HO +H 2O CH3 HO O O HO O CH3 O HO OH O OH NH2 NH2 OH OH HO O O H H OH HO O O HO O CH3 H HO O O O +2[H] CH3 N(CH3)2 OH H +[NH2 ], +2[CH3 ] OH OH NH2 NH2 OH OH HO Cl CH3 H HO O O HO O HO O O O [Cl] N(CH3)2 OH H3C OH R H NH2 OH HO HO O O O N(CH3)2 OH A B C OH O OH D CONH2 OH Cl H C 3 OH H O R=H : tetracycline R=OH : terramycin N(CH3)2 OH A B C OH O OH D OH aureomycin CONH 5. Polyketides (Dayrit) 2 O 52 +2[H] [O] [CH 3] CH3 OH OH o o o o o NH2 o o o o CONH2 HO CH3 H Biosynthesis of tetracyclines from Streptomyces species. HO OH O [O] CH3 O H OH O OH OH NH2 NH2 HO +H 2O CH3 HO O O HO O CH3 O HO OH O H OH NH2 NH2 OH OH HO O O H OH HO O O HO O CH3 H HO O O O +2[H] CH3 N(CH3)2 OH H +[NH2 ], +2[CH3 ] OH OH NH2 NH2 OH OH HO Cl CH3 HO O O O [Cl] 5. Polyketides (Dayrit) H N(CH3)2 OH HO HO O O 53 O HO +H 2O CH3 HO O O HO O CH3 O HO OH O H OH NH2 NH2 OH OH HO O O H OH HO O O HO O CH3 H HO O O O +2[H] CH3 N(CH3)2 OH H +[NH2 ], +2[CH3 ] OH OH NH2 NH2 OH OH HO Cl CH3 H HO O O HO O HO O O O [Cl] N(CH3)2 OH H3C OH R H NH2 OH HO HO O O OH A O N(CH3)2 B C D CONH2 OH OH Cl H C 3 OH H O OH O R=H : tetracycline R=OH : terramycin N(CH3)2 OH A B C aureomycin D CONH 2 5. Polyketides (Dayrit) OH OH O OH O 54 Summary FAS and PKS probably share an evolutionary history. Like the fats, polyketides also arise from polymerization of acetyl CoA. The key features and steps are: 1. Alternative starter units are used, in particular in the formation of tetracyclic antibiotics and macrocylic lactones. 2. No reduction of the carbonyls, or reduction to alcohol level only. 3. Cyclization via Claisen displacement or aldol reaction. There are many modes of cyclization depending on the chain length. 5. Polyketides (Dayrit) 55 Summary 4. Aromatization often follows with loss of H2O. 5. wider range of compounds are produced: macrocyclic lactones, phenols, quinones, and polycylic aromatic compounds. 6. Polyketides are attractive research targets because of their strong and varied biological activity, the modular nature of the genetic system and polyketide synthases, and relatively accessible biosynthetic expression systems. 5. Polyketides (Dayrit) 56