File
advertisement

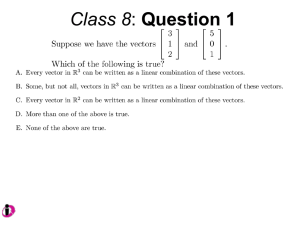
Unit I Lecture 1 B. Tech. (Biotechnology) III Year V th Semester EBT-501, Genetic Engineering EBT 501, Genetic Engineering Unit I Gene cloning -concept and basic steps; application of bacteria and viruses in genetic engineering; Molecular biology of E. coli and bacteriophages in the context of their use in genetic engineering, Cloning vectors: Plasmid cloning vector PBR322, Vectors for cloning large piece of DNA; –Bacteriophage-l and other phage vectors; Cosmids, Phagemids; YAC and BAC vectors, Model vectors for eukaryotes – Viruses, Unit II Restriction modification, enzymes used in recombinant DNA technology endonucleases, ligases and other enzymes useful in gene cloning, PCR technology for gene/DNA detection, cDNA, Use of Agrobacterium for genetic engineering in plants; Gene libraries; Use of marker genes. Cloning of foreign genes: DNA delivery methods -physical methods and biological methods, Genetic transformation of prokaryotes: Transferring DNA into E. coli –Chemical induction and Electroporation, Unit III Gene library: Construction cDNA library and genomic library, Screening of gene libraries – screening by DNA hybridization, immunological assay and protein activity, Marker genes: Selectable markers and Screenable markers, nonantibiotic markers, Gene expression in prokaryotes: Tissue specific promoter, wound inducible promoters, Strong and regulatable promoters; increasing protein production; Fusion proteins; Translation expression vectors; DNA integration into bacterial genome; Increasing secretions; Metabolic load, Recombinant protein production in yeast: Saccharomyces cerevisiae expression systems; Mammalian cell expression vectors: Selectable markers; Unit IV Origins of organismal cloning in developmental biology research on frogs; nuclear transfer procedures and the cloning of sheep (Dolly) & other mammals; applications in conservation; therapeutic vs. reproductive cloning; ethical issues and the prospects for human cloning; Two-vector expression system; two-gene expression vector, Directed mutagenesis; transposon mutagenesis, Gene targeting, Site specific recombination Unit V General principles of cell signaling, Extracellular signal molecule and their receptors, Operation of signaling molecules over various distances, Sharing of signal information, Cellular response to specific combinations of extracellular signal molecules; Different response by different cells to same extracellular signal molecule, NO signaling by binding to an enzyme inside target cell, Nuclear receptor; Ion channel linked, G-protein- linked and enzyme-linked receptors, Relay of signal by activated cell surface receptors via intracellular signaling proteins, Intracellular signaling proteins as molecular switches, Interaction between modular binding domain and signaling proteins, Remembering the effect of some signal by cells. Unit I • Gene cloning -concept and basic steps • Application of bacteria and viruses in genetic engineering • Molecular biology of E. coli and bacteriophages in the context of their use in genetic engineering • Cloning vectors: Plasmid cloning vector PBR322, • Vectors for cloning large piece of DNA – – – – Bacteriophage-l and other phage vectors Cosmids Phagemids YAC and BAC vectors • Model vectors for eukaryotes - Viruses, What is Gene Manipulation? Creation and cloning of r-DNA • r-DNA – Artificially created DNA molecule which bring together DNA Sequences not usually found together in nature • Gene Manipulation – Any, of variety of sophisticated techniques for creation of r-DNA and (in many cases) its subsequent introduction in living cell • Cloning – Propagation of r-DNA inside a particular host, so that many copies of same sequence are produced Cloning Overview Four main steps in cloning: •Insert synthesis •Restriction enzyme digest •Ligation •Transformation + Plasmid (vector) Insert (your gene) Functional construct Vectors Plasmid Vectors Bacteriophage Vectors Cosmid Vectors Shuttle Vectors Eukaryotic Vectors Yeast Artificial Chromosomes (YACs) Bacterial artificial chromosomes (BACs) Bacteriophage P1 artificial chromosomes (PACs) • Plasmid Vectors – pBR322, pSC101, pUC 18/19, pGEM3Z – Yeast Plasmid vectors (Yep, YRp, YCp, Yip) • Bacteriophage Vectors – λ phage vectors, phage M13 vectors, • Cosmid Vectors – pJB8 • Phagemid Vectors – pBluescriptSK(+/-) • Phasmid Vector – Phasmid λ ZAP vector • Artificial Chromosome Vectors – Bacterial artificial chromosomes (BACs) • pBeloBAC11 – Fosmid vectors – Yeast Artificial Chromosomes (YACs) • YAC3 – Bacteriophage P1 artificial chromosomes (PACs) • • • • Eukaryotic Vectors Shuttle Vectors Animal vectors Plant vectors Vectors Plasmid Vectors • Circular, double-stranded circular DNA molecules present in bacteria. • Range from 1 kb to over 200 kb. • Replicate autonomously. • Many carry antibiotic-resistance genes, which can be used as selectable markers. • Many useful cloning vectors were derived from plasmid pBR322. Plasmids • Plasmids are widely used as cloning vehicles • Plasmids are widely distributed throughout the prokaryotes Plasmids are replicons which are stably inherited in an extrachromosomal state • Plasmids to which phenotypic traits have not yet been ascribed are called cryptic plasmids • Most plasmids exist as double-stranded circular DNA molecules • If both strands of DNA are intact circles the molecules are described as covalently closed circles or CCC DNA • If only one strand is intact, then the molecules are described as open circles or OC DNA • Addition of an intercalating agent, such as ethidium bromide, to supercoiled DNA causes the plasmid to unwind. • If excess ethidium bromide is added, the plasmid will rewind in the opposite direction • Linear plasmids have been found in a variety of bacteria, e.g. Streptomyces sp. and Borrelia burgdorferi. • To prevent nuclease digestion, the ends of linear plasmids need to be protected, and two general mechanisms have evolved. • Either there are repeated sequences ending in a terminal DNA hairpin loop (Borrelia) or • the ends are protected by covalent attachment of a protein (Streptomyces). • Two major types – • conjugative or non-conjugative – depending upon whether or not they carry a set of transfer genes, called the tra genes, which promote bacterial conjugation • On the basis of their being maintained as multiple copies per cell (relaxed plasmids) or as a limited number of copies per cell (stringent plasmids) copy number of a plasmid is determined by regulating the initiation of plasmid replication • regulation by antisense RNA and regulation by binding of essential proteins to repeated sequences called iterons Plasmid Vectors • A plasmid is a genetic element that can replicate independently of the main chromosome in an extrachromosomal state. • Most plasmids are not required for the survival of the host cell. • Plasmids in E. coli – F Factor (Fertility Factor) – R Plasmids (Resistance Plasmids) – Col Plasmids (synthesize compounds that kill sensitive cells) Features of many modern Plasmids •Small size or low molecular weight plasmid •Origin of replication •Multiple cloning site (MCS) •Selectable marker genes •Some are expression vectors and have sequences that allow RNA polymerase to transcribe genes •DNA sequencing primers Essential Features of a Cloning Vector • Origin of replication – essential for selfreplication in host cells • Dominant selectable marker gene – usually confers drug resistance • One or more unique restriction sites A Polycloning Site is a Cluster of Unique Restriction Sites pBR322 Essential Features pBR322 Essential Features • pBR322 is early example of a widely used, purpose-built cloning vector • pBR322 contains the ApR and TcR genes of RSF2124 and pSC101, respectively • Replication elements of pMB1, a Col E1-like plasmid • completely sequenced with 4361 bp • it is totally characterized in terms of its restriction sites such that the exact length of every fragment can be calculated. These fragments can serve as DNA markers for sizing any other DNA fragment in the range of several base pairs up to the entire length of the plasmid • There are over 40 enzymes with unique cleavage sites on the pBR322 genome (Fig. 4.8). • The target sites of 11 of these enzymes lie within the tetracycline resistant (TcR) gene, and there are sites for a further two (ClaI and HindIII) within the promoter of that gene. • There are unique sites for six enzymes within the ampicillin resistant (ApR) gene. • Thus, cloning in pBR322 with the aid of any one of those 19 enzymes will result in insertional inactivation of either the ApR or the TcR markers. • However, cloning in the other unique sites does not permit the easy selection of recombinants, because neither of the antibiotic resistance determinants is inactivated. Selectable Markers • The commonest selectable markers are ones encoding resistance to antibiotics such as ampicillin (ApR), chloramphenicol (CmR), tetracycline (TcR), streptomycin (SmR), and kanamycin (KmR). • Another type of positive selection is reversal of auxotrophy. • For example, if the hisB+ gene is cloned in a vector then it is easy to select recombinants by transforming a hisB auxotroph and growing it in a medium lacking histidine. pBR322 Essential Features • Plasmid pBR322 has been a widely used cloning vehicle. • In addition, it has been widely used as a model system for the study of prokaryotic transcription and translation, as well as investigation of the effects of topological changes on DNA conformation. • The popularity of pBR322 is a direct result of the availability of an extensive body of information on its structure and function. • A large number of improved vectors have been derived from pBR322 eg. pBR325, pUC vectors • pBR325 encodes chloramphenicol resistance in addition to ampicillin and tetracycline resistance and has a unique EcoRI site in the CmR gene pUC vectors • use of polylinkers or multiple cloning sites (MCSs) • An MCS is a short DNA sequence, carrying sites for many different restriction endonucleases. • An MCS increases the number of potential cloning strategies available by extending the range of enzymes that can be used to generate a restriction fragment suitable for cloning • The pUC vectors also incorporate a DNA sequence that permits rapid visual detection of an insert. • The MCS is inserted into the lacZ′ sequence, which encodes the promoter and the α-peptide of β-galactosidase. • The insertion of the MCS into the lacZ′ fragment does not affect the ability of the α-peptide to mediate complementation, but cloning DNA fragments into the MCS does. The Blue-White Color Test • Therefore, recombinants can be detected by blue/white screening on growth medium containing Xgal • The E. coli lacZ gene encodes -galactosidase. -galactosidase converts the colorless substrate Xgal into a blue product. • Cells with -galactosidase activity produce blue colonies when grown on Xgal; cells lacking -galactosidase activity produce white colonies. Reporter Genes • Reporter genes are ones whose phenotype can be discerned by visual examination of colonies growing on a plate and/or ones that can be used to measure levels of gene expression. • In terms of analysis of recombinants, the most widely used reporter gene is the lacZ gene encoding b-galactosidase. As noted in Box 3.2, the presence or absence of b-galactosidase activity is easily detected by growing cells on medium containing the chromogenic substrate Xgal. • Another gene whose activity can be detected in a similar way is the gusA gene encoding b-glucuronidase. This enzyme cleaves 4-methylumbelliferyl-b-D-glucuronide (MUG) to release a blue pigment.