ECM Proteins_Dr. Jawad Hassan
advertisement

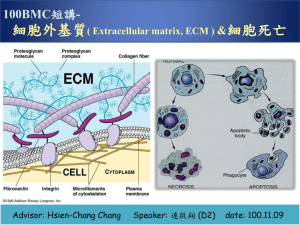
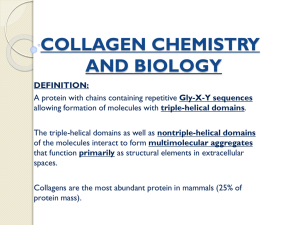
• Extra Cellular Matrix (ECM) • Structure, Synthesis, Function, Disease The Extra Cellular Matrix: ECM • Extra Cellular: outside the cell Matrix: structure made from a network of interacting components The ECM is composed of an interlocking mesh of fibrous proteins and glycosaminoglycans (GAGs). Components of the ECM are produced intracellularly by resident cells, and secreted into the ECM via exocytosis. Functions of ECM 1-Role in establishing and maintaining cell shape, migration, mechanical support 2-Anchorage for cells, segregating tissues from one another, and regulating intercellular communication 3-Sequesters a wide range of cellular growth factors, and acts as a local depot for them 4-Essential for processes like growth, wound healing etc What are the major proteins of the ECM? Collagens, Proteoglycans, Elastin, Fibronectin, Laminin, Tenascin. The collagens • A family of fibrous proteins found in all multicellular animals • They are secreted by connective tissue cells, as well as by a variety of other cell types • They are the most abundant proteins in mammals, constituting 25% of the total protein mass in these animals Structural features • Long, stiff, triple-stranded helical structure, • Three collagen polypeptide chains, called α chains, are wound around one another in a ropelike superhelix • A basic unit of mature collagen is called tropocollagen Composition of collagens • Collagens are extremely rich in proline and glycine • It is composed mainly of glycine (33%), proline (13%), 4-hydroxyproline (9%) • Hydroxyproline is unique for collagen and elastin Amino acid sequence • Every third residue is glycine which lies in the center of the triple helix, with the preceding residue being proline or hydroxyproline in a repetitive fashion – pro-Gly-X – hydroxypro-Gly-X Functions of amino acids • Proline stabilizes the helical conformation in each a chain • Glycine allows the three helical a chains to pack tightly together to form the final collagen superhelix Hydroxylysine • Collagen is also composed of hydroxylysine, which serves as attachment sites of polysaccharides making collagen a glycoprotein Lysine • Part of the toughness of collagen is accounted by the cross-linking of chains via lysine residues How? • Some of the lysine side chains are oxidized to aldehyde derivatives, which react with another lysine or another oxidized lysine via the action of lysyl oxidase Types of collagens • There are about 40 collagen genes dispersed throughout the genome and the protein products combine to form more than 28 different types of collagen. • The various collagens and the structures they form all serve the same purpose, to help tissues resist stretching. Classification of collagen 1. Fibril-forming collagens No interruptions in triple helix Regular arrangement results in characteristic “D” period of 67 nm Diameter : 50-500 nm Example : Types I, II, III, V, XI Classification of collagen 2. Network-forming collagens Forms network in basement (Collagen IV) and Descemet’s membrane (Collagen VIII) Molecular filtration Example : Types IV, VIII, X Classification of collagen 3. Fibril-associated collagens with interrupted triple helices (FACITs) Short collagens with interruptions Linked to collagen II and carries a GAG chain Found at the surface of fibril-forming collagens Example : Types IX, XII, XIV Classification of collagen 4. Anchoring collagens Provides functional integrity by connecting epithelium to stroma Example : Type VII Classification of collagen 5. Beaded-filament-forming collagens Form structural links with cells Example : Type VI Collagen VI crosslink into tetramers that assemble into long molecular chains (microfibrils) and have beaded repeat of 105 nm Synthesis of collagen • Individual collagen polypeptide chains are synthesized on membrane-bound ribosomes and injected into the lumen of the endoplasmic reticulum (ER) as larger precursors, called pro-a chains • In the lumen of the ER, selected prolines and lysines are hydroxylated to form hydroxyproline and hydroxylysine, respectively, and some of the hydroxylysines are glycosylated • Each pro-a chain then combines with two others to form a hydrogen-bonded, triple-stranded, helical molecule known as procollagen (Continued) Synthesis of collagen • During or following exocytosis, extracellular enzymes, the procollagen peptidases, remove the N-terminal and C-terminal propeptides • The resulting protein, often called tropocollagen (or simply collagen), consists almost entirely of a triple-stranded helix. • Excision of both propeptides allows the collagen molecules to polymerize into normal fibrils in the extracellular space Collagen-related diseases • Collagen is highly cross-linked in tissues where tensile strength is required such as Achilles tendon • If cross-linking is inhibited, the tensile strength of fibers is greatly reduced, collagenous tissues become fragile, and structures tend to tear (skin, tendon, and blood vessels) Diseases associated with collagen Diseases caused by mutations Subtypes of osteogenesis imperfecta (collagen I) Ehlers-Danlos syndrome (collagen I and V) Alport syndrome (collagen IV) Certain arterial aneurysms (collagen III) Ullrich muscular dystrophy (collagen VI) Certain chondrodysplasias (collagen IX and XI) Kniest dysplasia (collagen II) Scurvy • The formation of hydroxyproline requires vitamin C • Deficiency of vitamin C results in insufficient hydroxylation of proto-collagen and, hence, poor synthesis of collagen, formation of unstable triple helices preventing formation of normal fibrils • Non-hydroxylated procollagen degraded within the cell chains are then • This results in weakening of the collagen resulting in skin and gum lesions and weak blood vessels Types of OI • At least four types of osteogenesis imperfecta • Designated as type I through type IV • Type I osteogenesis imperfecta is the mildest form of the condition • Type II is the most severe results in death in utero or shortly after birth • Milder forms generate a severe crippling disease Mutations of OI • Mutations in the COL1A1 and COL1A2 genes cause OI • These mutations typically interfere with the assembly of type I collagen molecules • A defect in the structure of type I collagen weakens connective tissues, particularly bone, resulting in the characteristic features of OI • OI types I, II, and IV have an autosomal dominant pattern of inheritance, which means one copy of the altered gene in each cell is sufficient to cause the condition Chondrodysplasias • Mutations affecting type II collagen cause chondrodysplasias, characterized by abnormal cartilage, which leads to bone and joint deformities Ehlers-Danlos syndrome • A heterogenous group of disorders that affect connective tissues, which are tissues that support the skin, bones, blood vessels, and other organs • The signs and symptoms of Ehlers-Danlos syndrome vary from mildly loose joints to life-threatening complications Mutations in Ehlers-Danlos syndrome • Ehlers-Danlos syndrome results from defects in synthesis of either collagen molecules type I, III, or V or in the synthesis of collagen processing enzymes like procollagen Npeptidase, or lysyl hydroxylase resulting in mobile joints and skin abnormalities Non-collagen component of Bone Matrix Made up of stiffening substances to resist bending and compression (Inorganic matter ). The bone mineral is an analogue of crystals of calcium phosphate — hydroxyapatite Ca10(PO4)6(OH)2, a substance that can only be seen under electron microscopy. It is this association of hydroxyapatite with collagen fibres which is responsible for the hardness of bone. Elastin • The main component of elastic fibers is elastin • A highly hydrophobic protein, which, like collagen, is unusually rich in proline and glycine • But, unlike collagen, is not glycosylated • Contains some hydroxyproline but no hydroxylysine Formation of elastic network • Soluble tropoelastin (the biosynthetic precursor of elastin) is secreted into the extracellular space and assembled into elastic fibers close to the plasma membrane • After secretion, the tropoelastin molecules become highly cross-linked to one another, generating an extensive network of elastin fibers and sheets • The cross-links are formed between lysines by a mechanism similar to that of collagen molecules Elastin structure • The elastin protein is composed largely of two types of short segments that alternate along the polypeptide chain: – hydrophobic segments, which are responsible for the elastic properties of the molecule; and – alanine- and lysine-rich a-helical segments, which form cross-links between adjacent molecules Function of elastic fiber • Elastin is the dominant extracellular matrix protein in arteries • Mutations in the elastin gene causing a deficiency of the protein result in narrowing of the aorta or other arteries as a result of excessive proliferation of smooth muscle cells in the arterial wall • Apparently, the normal elasticity of an artery is required to restrain the proliferation of these cells Diseases of Elastic Fiber • • • • • • Cutis laxa Williams syndrome Buschke-Ollendorff syndrome Menkes disease Pseudoxanthoma elasticum, Marfan's syndrome – defects in copper metabolism (lysyl oxidase) Glycoproteins and Proteoglycans Glycoproteins Proteins conjugated to saccharides lacking a serial repeat unit Protein >> carbohydrate Proteoglycans Proteins conjugated to polysaccharides with serial repeat units Carbohydrate >> protein Glycosaminoglycans Mucopolysaccharides Glycoproteins • Proteins that contain oligosaccharide chains (glycans) covalently attached to polypeptide side-chains, in a cotranslational or posttranslational modification. • (N- Glycosylation), the addition of sugar chains can happen at the amide nitrogen on the side chain of the asparagine. • (O- Glycosylation), the addition of sugar chains can happen on the hydroxyl oxygen on the side chain of hydroxy-lysine, hydroxy-proline, serine, or threonine. Functions of Glycoproteins • • • • • • • Structural Reproduction Hormones Enzymes Carriers Inhibitors Immunological THANK YOU