CHEM-5398
April 1, 2010
Background: Steroids overview, etc
History
Estrogen
Synthetic Estrogens
Estrogen Antagonists/SERMs
Progesterone
Synthetic Progestins
Hormone Replacement Therapy (HRT)
Future Research…
Steroid hormones are all derived from
cholesterol
Cholesterol contains
cyclopentanophenanthrene ring
Estrogen and progestins are just two of
the many steroids found in the human
body
Cholesterol
Mechanism:
- Modulate gene expression inside cell
- They are not water-soluble so travel in
blood attached to protein carriers
- When they reach the cell, they
dissociate from protein carrier and enter
membrane
- Some bind to a receptor in the
cytoplasm and move in to the nucleus
Mechanism (ctd)
- Hormone binding activates receptor
protein and now both can bind specific
regions of DNA called HRE
(Hormone Response Elements)
Video
Ovarian and
Menstrual
cycles
and the
contraceptives
link
1926: Loewe and Lange discovered that a female sex
hormone varied throughout menstrual cycle.
1928: Zondek reported excretion of estrogen during
pregnancy.
In 1929 Adolf Butenandt and Edward Adelbert Doisy
independently isolated and determined the structure of
estrogen.
The first orally effective estrogen, Emmenin, was derived
from the late-pregnancy urine of Canadian women,
and was introduced in 1930
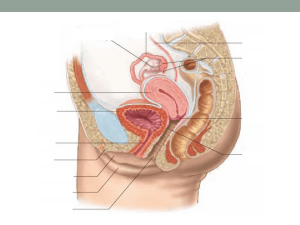
Function as the primary female sex
hormones
Present in both men and women
Promote development of female
secondary sex characteristics
Stimulate endometrial and uterine
growth
Reduce bone resorption, increase bone
formation
Fun fact: Estrus = fertile, gen = to
generate in Latin
Three major types of natural estrogens
Estrone (E1)
Estradiol (E2)
Most common!
Estriol (E3)
From menarche to menopause the
primary estrogen is 17β-estradiol
Estradiol is produced from testosterone
Estrogens act as signaling molecules by interacting
with specific target cells.
› Include tissues of the breast, uterus, brain, heart, liver, and
bone.
These target cells have estrogen receptors.
› There are two estrogen receptors that are normally found
in the cell’s nucleus: ER α and ER β.
The receptor undergoes dimerization in order for it
to have increased affinity for DNA.
This estrogen-receptor complex can now bind to
specific DNA sites, called estrogen response
elements (EREs).
Genes are activated to produce messenger RNA,
which guide the synthesis of new proteins,
determined by the cell type.
Menopause
Transition period in a woman's life when
her ovaries stop producing eggs, her
body produces less estrogen and
progesterone, and menstruation
becomes less frequent
Symptoms are mood swings, hot flashes
and vaginal dryness
Can by synthesized from plants or
biological organisms like horses
Examples include Synthroid or Quinestrol
Synthroid
Quinestrol
Estrogen Antagonists/SERMs
Estrogen antagonists are proteins that
block the actions of estrogen by binding
to estrogen receptors
As a result, estrogen can not bind
Example:
Evista/Raloxifene
SERMs
Selective Estrogen Receptor Modulators
Because Estrogen receptors differ slightly in
different organs, SERMs can target
receptors of a certain organ
So a SERM that blocks estrogen’s effects in
breast cells won’t impact estrogen binding
in the uterus!
Tamoxifen
Uses of SERMs..
Used before or after menopause
Can help in slowing metastasis of cancer
Can treat osteoporosis
Advantage: specificity
Yet to find a SERM that has no negative
side effect (delte this: both mentioned
cause colon cancer)
Progesterone/Progestins
Progesterone (pregn-4-ene3,20-dione)
History
1935: Progesterone is
discovered and named
1938: first orally active
progestin is synthesized in
Germany
1950s: More viable oral
progestin synthesized in
Mexico City by
Miramontes; approved in
US
Progesterone
Involved in female menstrual cycle,
supports pregnancy, and embryogenesis
in the womb
Synthesis:
Cholesterol
Pregnenolone
Progesterone
Progestins
Most frequent uses: Contraception and
endometrial hyperplasia
Enovid/Norethynodrel:
contraceptive
Progestin antagonists
When these bind receptors, they produce a
delay in endometrial maturation and
postpone the appearance of the
implantation window
Therefore, used to terminate pregnancies
Mifepristone
PRMs – Progesterone receptor modulators
(contraceptives)
Hormone Replacement
Therapy (HRT)
Estrogen + progestins or either!
Medical treatment for menopausal or
post-menopausal women
Progestins keep weight off and stop cell
proliferation
Benefits of estrogen:
› Reduction in loss of bone mass (osteoporosis)
› Decreased risk of cardiovascular disease
› Positive effect on cognitive function
Modes of HRT
Combination:
- Pills and patch
Estrogen:
- Pills, patch, cream
Progestins
- Pills, vaginal gels,
IUDs
Combination HRTs
Pills:
Prempro
Patches:
CombiPatch
Patches vs. Pills
Different routes of administration =
different side effects
Pills 2x likely to cause blood clots than
patches
Estrogen HRTs
Pills: Estrace
Patches: Vivelle-dot
Cream*: Dienestrol
Dangers of Estrogen Video
Dienestrol
Progestin HRTs
Pills:
Prometrium
IUDs:
Mirena (levonorgestrel)
Lasts up to 5 years
Negative effects of HRT
Mayo Clinic Research on HRT risks
In the largest clinical trial to date, the combination
estrogen-progestin (Prempro) increased the risk of
certain serious conditions.
According to the study, over one year, 10,000
women taking estrogen plus progestin might
experience:
- Seven more cases of heart disease than women
taking a placebo
- Eight more cases of breast cancer than women
taking a placebo
- Eight more cases of stroke than women taking a
placebo
- Eighteen more cases of blood clots than women
taking a placebo
Future research…
How testosterone and estrogen work together
to control male dimorphic behaviors in rats
(UCSF)
Alzheimer’s Disease and estrogen after
menopause
Assigned Reading:
Goodman and Gilman’s Pharmacological Basis
of Therapeutics pp. 1541and 1548-1568. Large
Print Only
Homework Questions:
1 What is a SERM? What are SERMs used for? Draw the structure of
tamoxifen.
2 What is Combipatch and how is it used? Draw the structures of the two
ingredients and list the general classes of molecules to which each of
these two ingredients belongs.
Sources
The Pharmacological Basis of Therapeutics by
Goodman and Gilman
“Progesterone vs Progestin” by Dr. Steven Hotze
“Perspective: Female Steroid Hormone Action” by
Dr. Orla Conneely
General, Sascha; Terebesi, Ildiko; Bracht, Stefan;
Funke, Adrian. Progestin -containing drug delivery
system. PCT Int. Appl. (2010), 77pp.
Daniels, Rolf. Estrogen drug delivery
systems. Pharmazie in Unserer
Zeit (2004), 33(5), 392-397.
Simmons, Horst Ernest. The Side Effects
of Estrogen Drug Therapy: Contraception and
Postmenopause. (1979), 92 pp.