Carbapenems

ANTIMICROBIAL RESISTANCE
9.21.12
Site of Action of antibiotics
• Inhibition of nucleic acid synthesis (Rifampin; quinilones)
• Inhibition of protein synthesis (Tetracyclines;
Chloramphenicol, macrolides, clindamycin, aminoglycosides, linezolid)
• Action on cell membrane (Polyenes; Polymyxin)
• Interference with enzyme system (Trimethoprim,
Sulphamethoxazole)
• Action on cell wall (Penicillin; cephalosporins, Vancomycin, carbapenams)
Mechanisms of Drug Resistance
• Change in drug target
• Production of an enzyme that modifies or inactivates the agent
• Reduced accumulation of the agent
• Limited uptake
• Active Efflux
• Loss of a pathway involved in drug activation
Mechanisms of Drug Resistance
Mechanisms of Drug Resistance
Mechanisms of Gram-Negative Bacterial
Resistance to Antibiotics
Antibiotic Class
Cephalosporins
-Lactamase inhibitors
Carbapenems
Mechanism of Resistance
ESBLs
chromosomal cephalosporinases
hyperproducers of -lactamases new -lactamases resistant to inhibitors
chromosomal cephalosporinases
porin mutations efflux pump overproduction (excluding imipenem) zinc metalloenzymes and other -lactamases
Fluoroquinolones
alterations in DNA topoisomerase
efflux mechanisms
permeability changes
Campaign to Prevent Antimicrobial Resistance in Healthcare Settings
Selection for antimicrobial-resistant
Strains
Resistant Strains
Rare
Antimicrobial
Exposure
Resistant Strains
Dominant
Target Alterations
• PBPs: in cell membrane
• S. pneumoniae, MRSA
• Intrinsic resistance, enterococci, gonococci, H. infl
• D-Ala-D-Ala target: VRE
• VanA, VanB, VanC, VanD
• Alterations in ribosomes
• Cell membrane changes
Protein Binding Proteins
• Target for all B-lactams
• found as both membrane-bound and cytoplasmic proteins
• all involved in the final stages of the synthesis of peptidoglycan, which is the major component of bacterial cell walls
• More common R mechanism for gram positive organisms
• Gram neg access to PBP is limited by outer membrane and thus other mechanisms supersede the binding to this target
Enzyme Production
• Aminoglycoside modifying enzymes
• B-lactamases:
•
•
•
Four structural classes:
• Class A: R of S aureus to penicillin, R of E coli to ampicillin and cephalothin –plasmid mediated
• Class B: hydrolyze carbapenmens/pens/cephs -chromosomal
Class C: chromosomal, active against cephalosporins
Class D: plamid mediatated
• ESBL: K. pneumoniae, E. coli : Derived from transfer of chromosomal genes for inducible amp C onto plasmids
B-lactamase
Cefipime
B-lactame ring
Increased stability to B-lactamase
Increased penetration into gram-positive
Ceftriaxone
-Lactamases: Overview
• Large, diverse family of enzymes
•
•
Widely dispersed in gram-positive (chromosoaml and plasmid) and gram-negative pathogens
(plasmid)
Major mechanism of resistance to
-lactams in gram-negative pathogens
• Wide range of activity: older enzymes hydrolyze older drugs, new derivatives have evolved for new drugs
•
• ESBLs
AmpC
-lactamases
• carbapenemases
-Lactamases
• Major groups for gram-neg
• TEM-wide spread-plasmid and transposon
• Enterobacteriaceae, Pseudomonas aeruginosa, Haemophilus influenzae , and Neisseria gonorrhoeae
• SHV-1
• Klebsiella pneumoniae (chromosomal) and E. coli (plasmid)
• Confer resistance to penicillins and first/second generation cephalosporins
-lactamase
Extended spectrum -lactamase
1960
TEM-1
TEM-2 SHV 1980s
Cefotaxime
TEM, SHV
CTX
ESBL-Mediated Resistance
• Contain a number of mutations that allow them to hydrolyze expandedspectrum β-lactam antibiotics
• Derived from older antibiotic-hydrolyzing
-
•
•
• lactamase enzymes (TEM-1, TEM-2, SHV-1)
• a single amino acid substitution can give rise to new
ESBLs
Not as catalytically efficient
Inhibited by β-lactamase inhibitors
Susceptible to cefoxitin and cefotetan in vitro only
• 10% –40% of K pneumoniae, E coli express
ESBLs
Rupp ME et al. Drugs.
2003;63:353 –365.
CTM-X predominant mechanism
E. Coli predominant organism
Canton, Cur Opin in Micr 2006, Pages 466–475
Coresistances among the Enterobacteriaceae isolates of the different ESBL types.
Morosini M et al. Antimicrob. Agents Chemother.
2006;50:2695-2699
Amp-C
• Confer resistance cephamycins (cefotetan, cefoxitin) and oxyimino- -lactams (cefotaxime, ceftriaxone, ceftazidime)
• Chromosomal in SPACE organisms and are inducible
• Poorly expressed in E. coli and is missing from klebsiella and salmonella species
• Plasmid mediated on other gram-neg, usually not inducible
• Not susceptible to inhibitors
AmpC- vs ESBL-Mediated Resistance
• Different phenotypic characteristics
• AmpC type
-lactamases typically encoded on chromosome of gram-negative bacteria, can also
• be found on plasmids
AmpC type
-lactamases hydrolyze broad- and extended-spectrum cephalosporins
• ESBLs —NOT AmpC
-lactamases —are inhibited by
-lactamase inhibitors (eg, clavulanic acid)
• AmpC production is less effective on cefipime so best cephalosporin to test
New CLSI Laboratory Standards
• Previously testing for ESBL was based on high MIC to oxyimino-beta-lactam substrates (cetriaxone, cefotaxime, cefipime, cetaz) and susceptibility to inhibitors followed by a confirmatory test to detect the enzyme
• Low sensitivity when mixed mechanisms at play, ie false positive results, some attempts to overcome this with cloxacillin-containing
Muller –Hinton agar, which inhibits AmpC activity
• When ESBL present susceptibility changed to resist for penicillins, cephalosporins and monobactams
• Current practice: MICs were changed
• 1-3 doubling dilutions lower
• No need for confirmation of enzyme
• No change in reporting
Epidemiology of Plasmid AmpC
Enzymes in the United States
• Alvarez et al examined a sample of 752 resistant
K pneumoniae , K oxytoca, and E coli strains from 70 sites in 25 US states
• Plasmids encoding AmpC-type
-lactamase were found in
• 8.5% K pneumoniae samples
• 6.9% K oxytoca samples
• 4% E coli samples
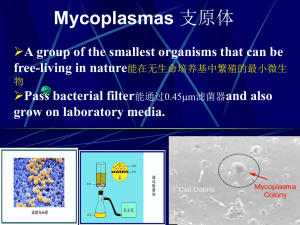
Carbapenemases
• beta-lactamases with versatile hydrolytic capacities.
• Ability to hydrolyze penicillins, cephalosporins, monobactams, and carbapenems.
• 2 major groups
•
• Metallo-b-lactamases (MBLs)
• Major R in pseudomonas, acinetobacter, and enterobacter
• Confer High level of R
Serine b-lactamases
• Oxacillinases or D b-lactamases (OxaA)
•
Not as Diverse
• Found mostly in acinetobacter
•
Confer only low level of hydrolytic activity therfore another R is necessary to raise MIC
• Class A carbapenemases
• Found in pseudomonas and enterobacter, but predominant type is found on a plasmid in Klebsiella
Mechanisms of Bacterial
Resistance to Fluoroquinolones
• Mutations in DNA gyrase and topoisomerase
• Overexpression of efflux pump system
• Bacterial membrane permeability changes
Mechanisms of Antibiotic Resistance in
Nonfermenters
• P aeruginosa and Acinetobacter often multidrug resistant 1
• Mechanisms of resistance include 1,2
• production of ESBLs or AmpC
-lactamases
• increased efflux of antibiotic agent
• decreased outer membrane permeability
• DNA gyrase mutations
• aminoglycoside modifying enzymes
Carbapenems: Resistance Issues
• Mechanisms of resistance to carbapenems in
P aeruginosa involve
•
• loss of OprD protein (initially called D2 porin) overproduction of efflux pump system
(MexA-MexB-OprM)
• upregulation of other efflux system may be involved (crossresistance to fluoroquinolones)
• Resistance to meropenem depends on both
• Resistance to imipenem mainly mediated through loss of OprD
Carbapenems: Resistance Issues
Ertapenem
Carbapenem nucleus
Mutated or missing
D2 porin
Imipenem
D2 Porin (OprD)
Outer membrane
Periplasm
Cytoplasmic membrane
PBP
1
Courtesy of John Quinn, MD.
PBP
2
PBP
3
PBP
4
Penicillin-binding proteins (PBPs)
PBP
5
Mechanisms of Carbapenem Resistance:
Impermeability
• OprD forms narrow transmembrane channels that are normally accessible only to carbapenems, not to other ß-lactams
• Loss of OprD porin is associated with decreased permeability of carbapenems and increased carbapenem MICs, whereas other ß-lactams remain active
Mechanisms of Carbapenem Resistance: Efflux
Systems in P aeruginosa
• Upregulation of MexAB-OprM efflux system
• associated with increased MICs of meropenem, not imipenem
• Coregulation of MexE-MexF-OprN efflux system with OprD porin in P aeruginosa
• upregulation of efflux associated with OprD
• associated with increased MICs of fluoroquinolones as well as carbapenems
• mechanism sometimes selected by fluoroquinolones, rarely by carbapenems
MRSA
• Methicillin resistance is acquired via Mec A
• mobile chromosomal element called staphylococcal cassette chromosome (SCCmec)
• SCCmec types I, II, and III and are multidrug resistant-large cassettes
•
Health-care associated
• SCCmec type IV and type V not multidrug resistant
• Community associated
MecA
• Encodes penicillin binding protein (PBP) 2a
• Weak affinity for methicillin and all beta-lactams
• Substitutes for the usual PBP 1-3 that have a high affinity for betalactams
• Speculation of origination from CoNS
S. Pneumoniae
• Pencillin
• Decreased affinity to PBP
• Can be overcome with high dose
• Macrolides
• Genetic changes to binding target on ribosome-high level can not be overcome =erm(B)
• Efflux pump-lower level-may be overcome =mef (A)
• Clindamycin
• Ribosomal methylation changing target erm(B)
S. pneumoniae
• Fluoroquinilones
•
• Bind to either gyrase or topoisomerase or both
Resistance from mutations in gyrA or parC
• reduce binding of the drug to the site of activity
• Mutations are step wise
•
One mutation and R to cipro and levo
•
More than one needed for gemi and moxi
• Tetracyclines
• Proteins are produced that package the drug into vessicles which are extruded from the cell
Enterococcus
• Intrinsic (chromosomal, naturally occurring) resistance to
• B-lactam
• 10 to 1000 times more drug to inhibit an average Enterococcus than an average Streptococcus
• Due to penicillinase production and PBP5 production
• Aminogylcosides
• Low level to streptocmycin and gentimicin
• Synergism causes cell wall agent to become bactericidal
• High level to tobramycin
Enterococcus-Intrinsic
• Clindamycin-gene encoding efflux pump
• TMP-SXZ-
• In vitro appears susceptible but in vitro is resistant
• Can utilize preformed folic acid
• Vancomycin at low levels in some strains
Enterococcus
• Genetic transfer to acquire new resistance
• One mechanism, involving pheromone-responsive plasmids, causes plasmid transfer between E. faecalis isolates at a very high frequency .
• Another mechanism involves plasmids that can transfer among a broad range of species and genera, although usually at a moderately low frequency .
• A third mechanism (conjugative transposition) involves transfer of specialized transposons at low frequency but to a very broad range of different kinds of bacteria .
Conjugative transposons are relatively nonselective in their host range and are one of the few types of elements known to have crossed the gram-positive/gram-negative barrier in naturally occurring clinical isolates and to then cause resistance in these various hosts
Enterococcus
• Acquired
• High level resistance to amnioglycosides
• Loose synergy ability as well
•
•
High level vancomycin resistance
• Van gene clusters on transposons or plasmids
•
Very old, probably initially resulted from pressor from natural glyocpeptides
•
Van A is the most common and confers highest level of resistance
Variable level to linezolid
• Depends on the number of mutations in the 23S rRNA