14 Hox genes
advertisement

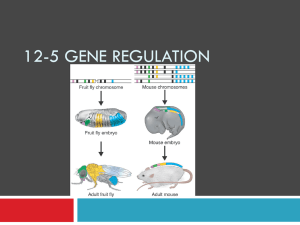
Hox genes and pattern development of vertebrates Pattern formation: harmonious arrays of different elements, such as the array of fingers on the hand, body pattern (head, trunk, and tail), or limb patterns. Pattern formation is best understood in Drosophila, where most genes that contribute to the body plan are described. Anteroposterior axis in vertebrates is specified by a group of genes called homeobox genes. There are many similarities to Drosophila. The dorsoventral axis in vertebrates is also specified by genes that have counterparts in Drosophila. Interestingly, although vertebrates and invertebrates share a similar body plan, it is inverted. Vertebrate limb development is controlled by multiple genes, including homeobox genes. Development depends on the process of reciprocal interaction between ectoderm and mesenchyme. Homeobox genes The homeobox: many genes that control pattern formation share a consensus sequence of 180 nucleotides. Genes with a homeobox are highly conserved in plants and animals and are called homeobox genes. Homeodomain: the protein encoded by a homeobox gene contains a 60 amino acid domain encoded by the homeobox. All homeodomain containing proteins are transcription factors that bind to gene specific promoters or enhancers. The homeodomain has three alpha helices. The recognition helix (a3) aligns in the major groove of DNA TALE proteins: (Exd) an atypical family of homeodomain proteins that help to target specific homeodomain transcription factors to their correct cis elements. They bind to DNA first, they alter the DNA conformation slightly, and this assists in binding of other homeodomain transcription factors. TALE proteins also stabilize bound homeodomain proteins. Homeobox genes are often clustered on one chromosome Homeobox genes exhibit two patterns of localization: 1. Some are scattered throughout the genome. 2. Hox genes: other homeobox genes are clustered within a small region and in a very specific sequence (Hox genes form a Hox complex). These Hox genes are highly conserved in organisms ranging from flies to humans. How did Hox genes evolve? Hox complexes: arose by repeated duplication and mutation of an ancestral homeobox gene. This formed an ancestral HOX complex. In some organisms, including most vertebrates, the HOX complex has been duplicated four times. Hox genes are organized into paralogy and orthology groups Paralogy group: are simply the natural clusters of Hox genes on the chromosome. Vertebrates have 4, flies have 1. They are named a, b, c, and d. Orthology group: sequence comparisons of each gene shows that certain genes are closely related. These are the genes in most vertical columns (for example, Hox a9, b9, c9 and d9). Orthologous Hox genes from different species can replace one another in function (Hox d4 substitutes for Dfd in Drosophila). Genes of any orthology group have corresponding positions within each complex (if clusters are aligned in rows, the orthology groups form columns. Genes are numbered consecutively from 113. Hox genes specify anteroposterior body pattern The physical order of Hox genes within the complex is related to their order of expression along the anteroposterior axis of the embryo!! Genes at the 3’ end are expressed in the anterior and genes at the 5’ end are expressed progressively further posteriorly. Hoxb cluster is expressed in the central nervous system. Each gene is first expressed at a sharply defined point and expression continues posteriorly and gradually tapers off. Rhombomeres: the pattern of Hox gene expression often coincide with repetitive bulges in the sides of the rhomencephalon. mouse What accounts for the pattern of Hox gene expression? mouse Enhancer sharing: the sequential order of Hox genes within the complex may result from the fact that all genes in the complex share a common enhancer element. If any gene is removed, or if the complex is broken apart, the removed genes may not be expressed properly. Thus, the sequential order has been preserved during evolution of different organisms. The dorsoventral body plan: are frogs just upside down flies? The dorsoventral body pattern of vertebrates and invertebrates are similar but inverted. Invertebrates, such as lobsters and flies, have the central nervous system on the ventral side and the heart is positioned dorsally. How is the dorsoventral axis established? Vertebrates: goosecoid stimulates noggin and chordin, which help induce the dorsal pattern. BMP-4 and xolloid induce the ventral pattern. Invertebrates: decapentaplegic (dpp) and tolloid induce dorsal development such as heart. Short gastrulation protein (sog) induces ventral development of CNS. Dpp and BMP-4 are similar and belong to the TGF-b family. Sog and chordin also share sequence similarity. Are sog and chordin interchangable in flies and frogs? If the body pattern of flies and frogs is established by similar signals, one might expect ectopic expression of sog from flies to act as Spemann’s organizer and to be able to organize neural tube in a frog embryo. Injection of sog RNA into the early frog gastrula causes formation of a second blastopore. Frog embryos can be completely ventralized by exposure to UV radiation (no CNS develops). However, the development of UV-treated embryos can be completely rescued by injection of sog RNA. Injection of noggin RNA from frogs into Drosophila embryos prevented the development of normal dorsal structures such as amnioserosa and dorsal ectoderm, but induced a second set of neurons. Thus, the signals for dorsoventral pattern formation are similar in flies and frogs. However, it is a mystery why they become inverted. Hox gene expression contributes to limb development Limb buds: the first stage of limb development occurs at 5 weeks in humans when small paddle shaped limb buds form. Apical ectodermal ridge (AER): a specialized structure formed by the ectodermal covering of the limb bud. It is a ridge that runs anterior to posterior. Progress zone: underneath the AER lies a zone of mesenchyme that actively proliferates to form the limb. Somites contribute mesenchyme to form muscles and lateral plate mesoderm forms cartilage and connective tissue. The limb is formed by differential growth of mesenchyme cells, by programmed cell death (between digits), and specific patterns of differentiation induced by Hox genes and other factors. How does the limb know where to form? How do arms become different than legs? How does the limb develop its three axis? Limb position is determined by FGF and Hox genes The position of limb development depends on signals from other tissues. Fibroblast growth factor (FGF): these growth factors are produced by mesenchyme (FGF-10) and epidermis (FGF-8) to induce limb formation. If a small bead containing FGF is implanted under the skin, an extra limb develops. If the bead is implanted in the flank near the anterior, it forms a wing. If the bead is implanted posteriorly, it forms a leg. Knock out mice lacking FGF-10 fail to develop limbs and have no apical ectodermal ridge or zone of polarizing activity. Hox gene expression is directly changed by FGF beads. Under normal conditions, wing expresses Hox d9 Flank expresses Hox b9 and c9. Leg expresses Hox b9, c9, and d9. Ectopic development of wing on the flank results in loss of Hox b9 and Hox c9, whereas, ectopic development of leg is induced by induction of all three Hox genes. Signals from somites and lateral plate mesoderm specify the dorsoventral limb axis Limb bud mesoderm is first induced by signals from the adjacent somites. This mesoderm then induces ectoderm to form the AER. The somite continues to induce ectoderm to form the dorsal surface of the limb. The lateral plate mesoderm induces the ventral portion of limb ectoderm. Radical fringe: a gene expressed in the dorsal ectoderm that provides a dorsalizing function and helps to induce the AER. Wnt-7a: another gene expressed in the dorsal ectoderm. It stimulates the underlying mesenchyme to produce Lmx-1, an important signal for dorsal differentiation. Engrailed: a gene expressed in the ventral ectoderm that provides a ventralizing function. It also inhibits expression of radical fringe and Lmx-1. The AER develops at the junction between areas that express radical fringe and engrailed. Sonic hedgehog induces the anteroposterior pattern of fingers and toes If a small piece of the chicken posterior wing bud is grafted to the anterior portion of a second embryo, it causes extra digits in a mirror image pattern. Normally, II, III, and IV develop. Now IV, III, II, III, IV form. Zone of polarizing activity: the ability of a tissue to establish the posterior position of the anteroposterior pattern (rear of limb bud). Sonic hedgehog: shh is expressed at high level in the zone of polarizing activity, suggesting it’s importance. To test this, purified shh DNA was transfected into cells and the cells were transplanted into the wing bud. The exact same result occurred, thus, shh is important for generating wing pattern. Reciprocal interaction is critical for wing development Interactions occur between the AER on the surface and limb bud mesenchyme that lies underneath in the progress zone. Limb bud mesenchyme induces formation of the AER from limb bud ectoderm. If the ectoderm of a limb bud is removed at an early stage of development, the mesoderm induces a new AER. If the AER is removed during a later stage, the limb mesenchyme stops growing and the limb is truncated. If the limb bud mesenchyme is removed or replaced with other tissue, the AER quickly degenerates. The AER serves a permissive rather than an instructive role. If you reverse its orientation, digits will develop normally. If you replace wing AER with leg AER, the wing develops normally. The mesenchyme is the instructive influence for limb development.