Regulation of Chloroplast Gene Expression
advertisement

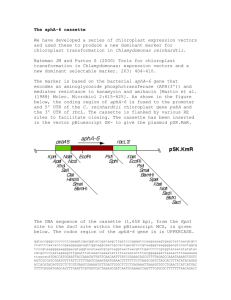
Regulation of Chloroplast Gene Expression • Studied principally during photomorphogenesis – i.e., development of cotyledons and leaves during "greening" (etioplast -> chloroplast). • Also studied (in mature chloroplasts) during light-dark cycles, and in response to certain stresses (heat, cold, radiation). • Multiple levels are regulated for most genes. • Difficult to generalize, but some trends emerge. Plastid Transcriptional Regulation • Transcriptional regulation is often global or large-scale – NEP functions early in development, PEP dominates later (etioplast chloroplast) – PEP-transcribed genes increase or decrease together • • E.g. - overall transcription increases during "greening", but decreases during chloroplast chromoplast There are examples of gene-specific transcriptional regulation – psbD/psbC promoter switching in response to light Plastid development is plastic, mostly under nuclear control. Shoots: NEP proplastids PEP light etioplasts chloroplasts Declines chromoplasts Roots: More NEP, less PEP proplastids amyloplasts Need to express accD , and ycf1 and ycf2 in all organs/tissues, essential for growth. psbD-psbC Light-responsive Promoter (LRP or BLRP) ● Preferentially utilized in the light (not dark); stimulated by blue and UV light. ● Also shows circadian rhythm of utilization. ● Evolutionarily conserved among higher plants. ● PEP-type promoter, but the -35 region not necessary. ● ● 2 upstream regions important for the light-response: PGT and AAG boxes. Boxes bind proteins (PTF1, AGF); binding of PTF1 is inhibited by ADP-dependent phosphorylation (ADP levels increase in darkness). BLRP promoter psbD BLRP Schematic diagram of the barley psbD-LRP and constructs used for plastid transformation. A. The boxed regions identify conserved sequences which include the PGT-box (−71 to −100), AAG-box (−36 to −56) and the prokaryotic-like −10 (−7 to −12) and −35 (−28 to −33) promoter elements. The psbD open-reading frame is shown at the far right. The direction of transcription is represented as an arrow and the initiation site is labeled as +1. A sequence alignment between the barley (Sexton et al., 1990b) and tobacco (Shinozaki et al., 1986) psbD-LRP was made with the ClustalW 1.7 Multiple Sequence Alignment Program. Aligned nucleotide sequences corresponding to conserved sequences are boxed in and labeled accordingly. Numbering of nucleotides and designation of conserved promoter elements are in accordance with the structure of the barley psbD-LRP from the transcription initiation site (+1). (From Thum et al. 2001) Models of transcription complexes associated with the psbD BLRP, rbcL and psbA promoters - extra TATA box likely maintains high rate of transcription in mature chloroplast Kim, M. et al. J. Biol. Chem. 1999;274:4684-4692 Regulation of RNA splicing & stability 1. Splicing of psbA introns (Group I) in Chlamy is strongly promoted by light (& redox). 2. Splicing of some photosynthetic genes’ introns (Group II) is inefficient in maize roots (amyloplasts), but efficient in leaves. 3. Stability of some plastid mRNAs increases during greening (psbA), but most decrease in mature chloroplasts in the light. Light-Dependent Splicing of psbA psbAi2 intron LSU rRNA intron Translational Regulation • • Cp mRNAs are relatively long-lived (half lives of 0.5 to 8 h or more) Translation is regulated by: 1. Global changes in rate (e.g., light-dark cycles) e.g. - high in daytime, low at night 2. Preferential translation of specific mRNAs under certain conditions. e.g.- very high light intensity increases psbA translation and decreases rbcL translation Light-activated translation of psbA mRNA • Complex of proteins that bind to the 5’ UTR of psbA mRNA in the light. • Demonstrate with gelshift (electrophoretic mobility shift) assay. Lane 1 – control (no protein extract) Lane 2 - extract from light-grown cells Lane 3 - extract from dark-grown cells S. Mayfield lab Box 9.4 in Buchanan et al. Proteins in complex that bind to the 5’ UTR of psbA mRNA 1. PABP - similar to a polyA-binding protein, binds A-U rich region in the 5’ UTR, activates translation 2. PDI - a protein disulfide isomerase (reduces disulfide bonds on certain proteins), activates PABP to bind RNA 3. Kinase – responds to ADP levels, at high [ADP], kinase deactivates PDI by phosphorylating it Model for Activation of psbA translation by Light via photosynthesis. Fig. 9.23 in Buchanan et al. Ribulose-1,5-bisphosphate carboxylase/oxygenase, RuBPCase (or Rubisco) • Catalyzes carboxylation of ribulose-1,5 bisphosphate: CO2 + RuBP 3PGA (x 2) • 2 subunits, large (LS) and small (SS) – 8 copies of each per holoenzyme • LS gene (rbcL) in the chloroplast • SS gene (rbcS) in the nucleus • extremely abundant, because inefficient – Pyrenoid in algae is mostly RuBPCase • regulated during light-dark cycles – enzyme more active in the light – also synthesized mainly in the light Translational regulation of RuBPCase LS by SS Incoming SS somehow promotes translation of rbcL mRNA! Bogorad lab Fig. 9.16 in Buchanan et al. There also seems to be autoregulation of rbcL translation: Cohen et al. (2006) Plant Physiol. 141, 1089; Wostrikoff and Stern (2007) PNAS 104, 6466 Rough Thylakoids • Polyribosome (polysomes) can be observed bound to thylakoid membranes. • At least some of these polysomes are attached to the membrane by the nascent (“new”) protein. • Suggests these polysomes make thylakoid membrane proteins and simultaneously insert them into the membrane. • Chloroplasts also contain a Signal Recognition Particle (SRP) homologue. Thylakoid-bound polysomes from Chlamydomonas Occur in the light period of a light-dark cycle polysome polysome A. Michaels, M. Margulies and G. Palade (~1972) Stabilization of nascent chlorophyll binding proteins of PSI and PSII with Chlorophyll Fig 9.24 in Buchanan et al. Regulation of protein stability in chloroplasts Protein stability is regulated by: 1. binding of cofactors (e.g., chlorophyll and carotenoids) 2. assembly with other subunits in a multi-subunit enzyme complex (PSI,PSII, ATP syn) ATP Synthetase Photoinhibition: inhibition of photosynthesis at very high light flux Photosystem II damage is critical. Box 9.6 in Buchanan et al. psbA encodes ~32-35 kDa D1 polypeptide of PSII Yamamoto, Plant Cell Physiol. 2001 D1 protein turns over rapidly because it becomes damaged in the light. D1 turnover and replacement is ongoing and critical • At photoinhibitory light intensity, D1 protein of PSII is damaged faster that it can be removed (and degraded). • At most lower light intensities, degradation, synthesis and replacement of D1 keeps up with the damage rate. Retrograde Signaling & Regulation Retrograde Regulation - Regulation of nuclear genes by the chloroplast - Nuclear genes typically encode chloroplast proteins - Signaled by: (1) Developmental state of the plastid/gene expression (2) Photo-oxidative stress Anterograde Regulation - Regulation of chloroplast genes by nuclear gene products - Occurs at most levels of expression GUN Genes and Retrograde Signaling Zhang,D (2007) Signaling to the nucleus w/a loaded GUN. Science 316, 700-701.