Chemistry in Focus 3rd edition Tro
advertisement

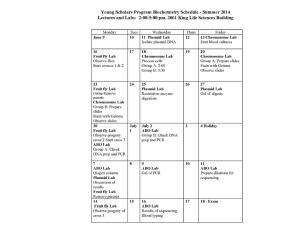
Chemistry in Focus 3rd edition Tro Chapter 16 Biochemistry and Biotechnology Brown Hair, Blue Eyes, and Big Mice • Study of the molecular blueprints that are genes has increased our understanding of how we think, how we behave, and what diseases we might develop. • We understand not only how a molecular sequence works, but how to take it from one organism and implant it in another. • 4 type of molecules in living organisms – – – – Lipids Carbohydrates Proteins Nucleic acids Lipids and Fats • Lipids are cellular components that are insoluble in water, but extractable in nonpolar solvents. – Fats, oils, fatty acids, steroids, some vitamins • They form the structural components of biological membranes and reservoirs for long-term energy storage. • They contain twice as much energy per gram than any other class of biochemical compounds. – Efficient energy storage Fatty Acids • One type of lipid • Organic acid with a long hydrocarbon tail • General formula RCOOH: Triglycerides • Fats and oils are a combination of glycerol and three fatty acids. Tristearin • Structure/property relationships – Long hydrocarbon chains: nonpolar, immiscible with water – Energy is extracted via oxidation of these long chains (as in gasoline). – Chains are saturated: efficient packing, solids – Fat is conveniently stored in the body. • Provides thermal insulation Triolein • Main component of olive oil • Double bonds in R groups interferes with efficient packing, liquid at room temperature Trilinolenin • Polyunsaturated fat: multiple double bonds in the hydrocarbon chains – Animal fats tend to be saturated. – Plant fats tend to be unsaturated. • Variations in structure serve different purposes in the human body. Carbohydrates • Chemical formulas are multiples of CH2O, carbon and water • Function in the body as short-term energy storage • Chemical structure related to: • Carbohydrates are polyhydroxy aldehydes, or ketones, or their derivatives. Glucose • This is a dynamic system, but at any instant more molecules are in the ring form. Glucose Properties • Hydroxyl groups mean strong hydrogen bonding with each other and with water. • Solubility in body fluids leads to function as a quick energy source. • Since it is partially oxidized, it yields less energy per gram than octane or lipids. • Balance between efficient energy storage and ease of access to that energy Fructose • Isomer of glucose • Two CH2OH groups mean it is more soluble in water and sweeter. – Takes less to offer same sweetness Saccharides • Monosaccharides – carbohydrates composed of a single ring • Disaccharides – joined monosaccharides, double ring structures Complex Carbohydrates • Polysaccharides – Most common are starch and cellulose – Subtle molecular difference (the oxygen linkage between rings and subsequent nature of resulting hydrogen bonds) means a dramatic macroscopic result. – Human enzymes cannot cut chains of cellulose. Proteins • The body CAN metabolize proteins. • The body metabolizes proteins ONLY as a last resort. • Proteins have much more important other work to do in the body. Protein Functions • Compose much of the physical structure of the body (muscle, hair, skin) • Act as enzymes to control chemical reactions • Act as hormones to regulate metabolic processes • Transport oxygen from lungs to cells • Act as antibodies • Protein molecules are long chains of repeating units of amino acids. – Differences among amino acids arise from different R groups. • Changing the number and order of these amino acids changes the functionality of the protein. • The simplest R group is the hydrogen atom, and the amino acid is glycine. The Peptide Bond • The acidic end of one amino acid reacts with the amine side of another to form a peptide bond. • Two linked amino acids is called a dipeptide. • Chains with 50 units or less are polypeptides; chains with over 50 units are called proteins. Sickle Cell Anemia • Hemoglobin (Hb) is a medium size protein with a molecular formula that contains close to 10,000 atoms: C2952H4664O832S8Fe4 • Replacing polar glutamate with nonpolar valine at one position, on two of these chains, lowers the solubility of Hb resulting in red blood cell deformation. Protein Structure • The structure of a protein is finely tuned to achieve a specific function. • We characterize protein structure in four categories: – Primary – Secondary – Tertiary – Quaternary Primary Structure • The amino acid sequence held together by peptide bonds • Abbreviations like gly-val-ala-asp are used to note the sequence of the amino acid. Secondary Structure • The way the amino acid chain orients itself along it axis – Alpha-helix – Pleated sheet Alpha-Helix • Helical shape is maintained by hydrogen bonds between different amino acids along the protein chain. • α-keratin is an alpha-helix and is responsible for the elasticity of hair and wool. • It works like a spring. Pleated Sheet • Protein forms zig-zag chains that stack neatly • Silk is pleated sheet • Inelasticity due to full extension of protein chains • Softness due to sliding of sheets past each other Tertiary and Quaternary Structure • Tertiary structure is the bending and folding due to interactions between amino acids on the chain. – Completely extended – Globular or ball-like • Overall shape of the particular protein strand • Arrangement of subunits of the protein chain in space is quaternary structure. Interactions of R Groups to Determine Tertiary and Quaternary Structure Common Proteins: Hemoglobin • Entire structure not known until late 1950s • HB folds to hold four flat molecules called heme groups. – Pick up oxygen at lungs – Release it at cells undergoing glucose oxidation • Interior of Hb molecule is highly nonpolar. – Repels water – Allows oxygen in and out • Exterior is polar – Hemoglobin is soluble in water. α-Keratin • Composes hair and wool • α-helix structure maintained by hydrogen bonding • Hair – 3 α-helices in a coil held by hydrogen bonds (easy to change) and disulfide linkages (require chemical treatment) Lysozyme • Acts as an enzyme • Cleaves polysaccharide units within cell walls – Walls explode killing the bacteria • In nasal mucus and tears • Discovered by Alexander Fleming in 1922 Insulin • Acts as a hormone • Synthesized in the pancreas • Small (51 amino acids) • Promotes entry of glucose into muscle and fat cells, lowering blood glucose level • Diabetics must inject insulin. Nucleic Acids • The templates from which all proteins are made • Two types – DNA (deoxyribonucleic acid) • Occurs in cell information center – RNA (ribonucleic acid) • Occurs throughout interior of cells Nucleotides Nucleotides • Phosphate and sugar groups are identical in every nucleotide. • Four different bases – – – – A, adenine T, thymine C, cytosine G, guanine • Codon – A group of three bases that codes for one amino acid • With minor exceptions, the code is universal; it is identical in all organisms, from bacteria to humans. DNA • Occurs in chromosomes, found in the nucleus of most cells of the human body – There are 46 in humans • Each set of DNA contains all the DNA required to specify an entire person. – Organs make those proteins specific for their own functioning. – But the blueprint is there for everything else too DNA Replication • Mechanism elucidated by Watson, Crick, and Franklin in 1953 • Complementary base units are formed (with the help of enzymes) after the double-helix unzips. – Two daughter DNA strands formed • Daughter DNA molecules are identical in every way to the parent. Protein Synthesis • Genes are sections of DNA, thousands of base pairs long. • When the gene for a protein is needed, that section of DNA unwinds. • A messenger RNA (mRNA) is formed, which is a complement to the unwound section. • expression • mRNA goes to a ribosome where protein synthesis occurs. • Cells express only the proteins specific to their function. Viruses • Definition lies somewhere between life and non-life. – Difficult to kill, do not respond to antibiotics • Require the machinery of a host cell to reproduce – Virus inserts it own DNA into the chromosomes of the host. – Host then expresses viral DNA • Common cold, flu, measles, polio, smallpox, ebola AIDS • HIV causes AIDS • HIV attacks immune system cells, releasing its RNA. • Reverse transcriptase forms viral DNA from the RNA • An enzyme inserts the DNA into the chromosomes of the host cell. • Cell dies, releasing daughter HIVs Recombinant DNA Technology • Employs restriction enzymes which cut DNA in specific places • DNA pieces can be separated by gel electrophoresis. – Even single genes can be isolated. • A DNA strand from one organism (a human) can be introduced into another (a bacterium). • Bacterium are cultured, replicating DNA. • This is a source for the protein coded for by that DNA. Pharmaceuticals • Insulin – Animal insulin is not tolerated by all diabetics. – The gene that codes for the production of human insulin was copied and expressed by a bacteria. – Human insulin factory – Most diabetics take genetically engineered insulin today. • Human growth hormone Agriculture • Bacteria, without the protein that accelerates ice crystal formation on crop leaves, have been engineered. • What impacts might this (and similar technologies) have on the environment? Genetic Screening and Disease Therapy • Can we screen for genes that may indicate predisposition to disease? – And should insurance companies have access to this information? • Genetic engineering techniques might one day be used to treat genetic disease directly. – CF, Huntington’s disease, MD CLONING • When egg DNA is modified, whole new organisms can develop. • Science fiction is now possible in reality. • Embryonic cloning has been achieved in animals. • By nuclear transfer, cloning of adult organisms has been achieved in animals. Therapeutic Cloning and Stem Cells • Reproductive cloning is generally viewed as unethical. • Therapeutic cloning is regarded as acceptable. – Goal is to produce embryonic stem cells that are genetically identical to the adult donor – These are the master cells normally present in embryos, days after the fertilization of an egg. • Therapeutic cloning offers the potential to make stem cells that are a perfect genetic match to the donor of the DNA from whom the stem cells are cloned. – No rejection by the immune system – Fraught with controversy