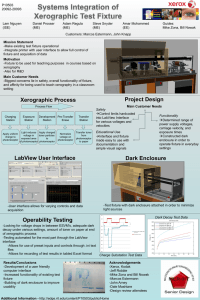
Proof of Principal, Medical Therapy and Clinical Trials
advertisement

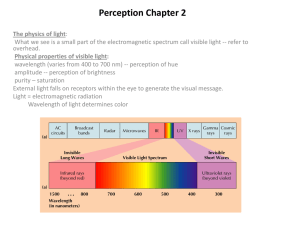
Congress Highlights: Progress in Retinal Degeneration Research From Scientific Darkness to the Light of Clinical Trials ohenetihool LGerald J. Chader, Ph.D., M.D.hc Doheny Eye Institute Los Angeles, CA USA University atlo os Angeles, CA October 22, 2009 Q.: WHAT ARE WE DOING TO FIND NEW RD TREATMENTS? Much Basic Science Progress Researchers have now established a firm basis in the genetics and cell biology of the RD diseases. • We know many of the genetic and protein mutations through genomics and proteomics. • We also know the biochemical pathway (apoptosis) that leads to photoreceptor cell death and we know several neuron-survival agents that slow apoptosis. • We have a much better idea of clinical and genetic diagnosis of RP types and how this relates to the use of therapeutics. Now, building on this basic information what potential treatments and cures for the RDs are here now or are coming? • But, before talking about specific treatments, we must first understand the 2 different disease situations that will determine which type of therapy might or might not be applied. The first disease situation is… When most or all of the photoreceptors are dead. Here we use treatments that replace the dead cells or at least replace their function in the retina. These could be: 1) Stem/Progenitor cell transplantation 2) Electronic Prosthetic Devices (Artificial Vision) 3) Optogenetics Optical Photoswitchs The second disease situation is… When at least some photoreceptor cells yet are alive. Here, we would use treatments that prolong photoreceptor life and make them function better such as: 4) Neuroprotection 5) Antioxidants Nutrition 6) Gene Therapy 1) Photoreceptor Transplantation • If photoreceptor cells are dead, why not just transplant normal photoreceptor cells into the RD retina from a normal donor retina? • Unfortunately, this has shown only limited success in many animal studies and even in a human clinical trial. • So, direct photoreceptor cell transplantation has yet to be proven to be effective for the human. 1) Alternative: Stem Cell Use • Stem cells are primitive, multipotential cells that have the ability to develop into all different adult cell types – such as photoreceptor cells. • So, stem cells transplanted into the retina might replenish the supply of photoreceptor cells that died due to Q.: But - Can We Actually Transform Stem Cells into Mature Photoreceptor Cells? In an important preclinical study, human embryonic stem cells transplanted into the retina of a mouse model with a form of LCA restore some visual function. A human clinical trial could result. Future Treatment? • Direct Photoreceptor cell transplantation: Not yet! • Embryonic Stem Cells → Photoreceptor Cells: good research is continuing. Many photoreceptor characteristics can be induced in the embryonic cells. • Research on other cell types is being conducted to see if they can be converted into mature photoreceptor cells. 2) Artificial Vision Uses an electronic prosthetic device to replace the function of dead photoreceptors. The design is simple: 1) External camera captures the light image 2) Computer processing 3) Electronic signal passes to an array of electrodes attached to remaining inner retinal cells in the eye. 4) Finally, the signal is passed down the optic nerve to the brain to produce a visual image. Clinical Trials? • Second Sight Medical Products implanted about 40 RP patients in Europe and the Americas. Good results have been reported in restoring at least some functional vision. Importantly, safety is very good. • Other groups such as in Ireland, Australia, etc. are doing great work. • Professor Zrenner with Retina Implants AG is doing excellent clinical work on another type of retinal device that should lead to a commercial product in the near future. Future Treatments? • Several groups are doing human testing including at least three companies. • SSMP has a prosthesis that has received the EAU CMark and is available for general implantation in RP patients in Europe. • Technologies are being improved to allow for face recognition and reading ability in the future. • Many animal and plant cells 3) Optogenetics have proteins that react to light and produce an electrical signal. • Molecular engineering can be used to insert channelrhodopsin or similar molecules into surviving retinal bipolar or Chlamydomonas is a ganglion cells in animals to tiny one-celled algae make them light sensitive. that contains a lightsensitive protein • These signals can be passed on called channel to the brain for light perception. rhodopsin Future Treatments? • Basic work on Photoswitches is yet at an early stage of development but is progressing rapidly. • Optogenetics could thus give functional vision using non-photoreceptor, secondary neurons of the retina. Remember that the second disease situation is…… when some living photoreceptors yet remain. Possible therapies would be: 4) Neuroprotection 5) Antioxidants Nutrition 6) Gene Therapy 4) Neuroprotection Neuroprotection is the use of special small molecules or electrical stimulation to protect photoreceptor cells and make them live longer. • Many natural factors (growth factors) in brain, retina and other tissues have been found that slow photoreceptor cell death. One is named CNTF. Another is the Rod-Derived Cone Viability Factor. • In other cases, small molecular weight drugs can make photoreceptor cells work better. Neuroprotection: Clinical Trials? • Neurotech is in clinical trials with CNTF on RP and dry AMD subjects. The CNTF is placed inside the eye and enters the retina where it helps to protect the sick photoreceptor cells. • A drug clinical trial using small molecules called retinoids looks successful in 2 specific types of LCA. • There are many types of Neuroprotection that can be used – some are general, some only for specific types of RP. Future Treatments? • The current Neurotech clinical trial has shown good results to date. • It thus should produce the first effective and generally available treatment for most forms of RP and dry AMD. • Other drugs as well as electrical stimulation can improve vision and be used in the future. 5) Antioxidants It is clear that antioxidants can slow disease progression in some patients with mid-stage dry AMD. This was proven in the AREDS clinical trials. In Retinitis Pigmentosa, two research groups – Drs. Van Veen and Campochiaro have demonstrated that antioxidants slow the course of retinal degeneration in RP animal models. This is specifically by inhibiting photoreceptor cell death through apoptosis. . Antioxidant Trial Dr. van Veen fed animals with retinal degeneration a special combination of antioxidants and slowed the degeneration process. Together, these antioxidants are called RetinaComplex. Ingredients: Lutein, zeaxanthin, alpha-lipoic acid, L-glutathione, extract of lycium barbarum (wolfberry) Based on this preclinical work, a small clinical trial in Spain has finished on RP and dry AMD patients. The results look good. More extensive trials are planned. Future Treatments? • First, the clinical trials such as on RetinaComplex must be completed. • In the future, there are many types of antioxidants that can be tested in RP animal models and then in the human. • Until then, take your mother’s advice – Eat your Vegetables! They contain many good antioxidants. 6) Gene Therapy • Gene Therapy is the replacement of defective mutated genes in living cells with new, normal copies of the gene. • The new gene will synthesize a normal protein that replaces the mutated or missing protein and restores photoreceptor cell function. • Long-term, positive effects of Gene Therapy in RP animal models have been shown even if treatment is done fairly late in the disease after significant photoreceptor loss. Gene Therapy Clinical Trials The new and exciting news is that Gene Therapy will not just slow down the RD disease process but it can restore some visual function in the human. • About 4 years ago, Dr. Robin Ali et al. started the first gene therapy clinical trial supplying a normal copy of the RPE65 gene to specific patients with LCA. Other groups soon started similar trials and the patients seem to be doing well with some restored vision. • The focus now is on early treatment, i.e., children as in the exciting work of Dr. Jean Bennett. • This success can now be used as a model for treatment of many other ocular diseases. Future Treatments? Now, trials are in progress or planned for: • Stargardt’s Disease • Several forms of LCA • Retinoschisis • Forms of Usher Syndrome • Choroideremia Also, for types of: dominant, recessive and X-linked RP. In Conclusion…. In the Past - No gene mutations for RP were known. We had little idea as to the mechanism of photoreceptor cell death and there were no agents know that could slow photoreceptor degeneration and death. Now – About one-half of the RP mutations are know. Much is known about the basic mechanism of photoreceptor cell death and how to inhibit it. Electronic implants are available to patients. Basic work in the fields of stem cell biology and in Optogenetics show promise for future treatment. Also, several Clinical Trials are in progress in Neuroprotection, Gene Therapy, Antioxidant Therapy that can actually improve vision. What’s in the future? I hope you agree that we are finally passing out of the time of scientific darkness and into the enlightened era of clinical trials. These should soon lead to many new therapies that will save and restore vision in RD patients.