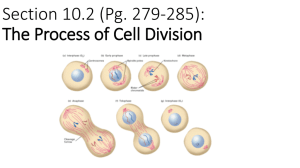
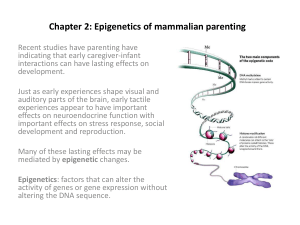
Document
advertisement

This slide series has been used for teaching to students of the Montpellier University in 2004. It explains basic features of the folding of DNA in nucleosomes and chromatin fibers, and discusses the regulatory roles of post-translational histone modificatons The material displayed is inspired from several sources. The literature used is cited within the slides. Moreover, several slides (indicated by the JHW logo) have been copied or modified from Jacob Waterborg, University of Missouri, Kansas City To see his chromatin material in full, see the website: http://sbs.umkc.edu/waterborg/ 10,000 nm DNA compaction in a human nucleus 11 nm 1bp (0.3nm) compact size DNA length compaction nucleus (human) 2 x 23 = 46 chromosomes 92 DNA molecules 10 m ball 12,000 Mbp 4 m DNA 400,000 x mitotic chromosome 2 chromatids, 1 m thick 2 DNA molecules 10 m long X 2x 130 Mbp 2x 43 mm DNA 10,000 x DNA domain anchored DNA loop 1 replicon ? 60 nm x 0.5 m 60 kbp 20 m DNA 35 x chromatin fiber approx. 6 nucleosomes per ‘turn’ of 11 nm 30 nm diameter 1200 bp 400 nm DNA 35 x nucleosome disk 1 ¾ turn of DNA (146 bp) + linker DNA 6 x 11 nm 200 bp 66 nm DNA 6 - 11 x 1 bp 0.33 nm DNA 1x base pair 0.33 x 1.1 nm Compaction of DNA by histones JHW Compaction by chromosome scaffold / nuclear matrix 3-99 Copyright 1999, J.H.Waterborg, UMKC 30nm Nuclear - chromosome compaction + 2M NaCl histones radial loop chrosomosome model Mitosis + 2M NaCl histones chromatid compact size DNA length compaction nucleus (human) 2 x 23 = 46 chromosomes 92 DNA molecules 10 m ball 12,000 Mbp 4 m DNA 400,000 x mitotic chromosome 2 chromatids, 1 m thick 2 DNA molecules 10 m long X 2x 130 Mbp 2x 43 mm DNA 10,000 x DNA domain anchored DNA loop 1 replicon ? 60 nm x 0.5 m 60 kbp 20 m DNA 35 x chromatin fiber approx. 6 nucleosomes per ‘turn’ of 11 nm 30 nm diameter 1200 bp 400 nm DNA 35 x disk 1 ¾ turn of DNA (146 bp) + linker DNA 6 x 11 nm 200 bp 66 nm DNA 6 - 11 x 1 bp 0.33 nm DNA 1x nucleosome JHW base pair Compaction by chromosome scaffold / nuclear matrix 0.33 x 1.1 nm 3-99 Copyright 1999, J.H.Waterborg, UMKC chromatid 1m mitotic chromosome 10 m DNA loops H1 HISTONES Linker histone are highly conserved, small, basic proteins H2A helix Core histones variable H3 H4 conserved Histone acetylation is a reversible modification of lysines in the N-termini of the core histones. Result: • reduced binding to DNA • destabilization of chromatin N JHW 3-99 Copyright 1999, J.H.Waterborg, UMKC H2B Core Histones The basic structure of ALL core histones is the same: • 1 long hydrophobic alpha-helix, bordered by • 2 short hydrophobic alpha helices that form pairs • H2A - H2B and H3 - H4 which interact. References: Moudrianakis et al. PNAS 88, 10138 (1991); PNAS 90, 10489 (1993); PNAS 92, 11170 (1995) JHW 3-99 Copyright 1999, J.H.Waterborg, UMKC The histone-fold H3-H4 tetramer JHW Histone octamer H2A-H2B dimer 3-99 Copyright 1999, J.H.Waterborg, UMKC Histone octamer assembly JHW Figure, courtesy from Timothy Richmond, ETH Zürich, Switzerland 3-99 Copyright 1999, J.H.Waterborg, UMKC The Nucleosome as the fundamental chromatin unit • 146-149 bp DNA in a 1.65 turns of a flat, left-handed superhelix • one pseudo twofold axis centered at the “dyad” (reference: 0 helical turns) • one base-pair precisely at the dyad • sharp bends at + 1.5 and + 4-5 turns • Histone-fod domains organize 121 bp of DNA. The DNA is bound at 10 bp intervals through many contacts, including penetration of arginines at all 14 minor grooves facing the protein core • The grooves from neighboring DNA turns line up; forming channels • H3 and H2B N-termini exit one of these channels every 20bp. • The H4 tail establishes contacts with the next core particle. JHW H2A H2B H3 H4 3-99 Copyright 1999, J.H.Waterborg, UMKC Nucleosome features < JHW 6 nm • Each core histone dimer has 6 DNA binding surfaces that organize 3 DNA turns; • The histone octamer organizes 145 bp of DNA in 1 3/4 helical turn of DNA: 48 nm of DNA packaged in a disc of 6 x 11nm > 3-99 Copyright 1999, J.H.Waterborg, UMKC < 11 nm > Histone octamer organizes 145 bp of DNA Luger, Mader, Richmond, Sargent & Richmond Nature 389, 251-260 (1997) JHW Question 1: What is the function of histone N-termini ? Question 2: Are all N-termini functionally equivalent ? 3-99 Copyright 1999, J.H.Waterborg, UMKC Where are the N-termini of the core histones ? H1/H5 globular protein domain Asymmetric between the DNA gyres ? On the OUTSIDE of the DNA gyres ? Zhou et al. Nature 395, 402 (1998) Wolffe et al. Science 274, 614 (1996) Linker histone H1 organizes exiting DNA (up to 168 - 200 bp). H1 stabilizes interaction between nucleosomes in compacted chromatin. JHW 3-99 Copyright 1999, J.H.Waterborg, UMKC Linker histone H1 C Chromatosome Core histone octamer + 1 Linker Histone + 2 full turns of DNA (168 bp) N H1 H3 1 mM 5 mM Linker Histone and histone termini control linker DNA entry/exit of chromatosome in chromatin fiber. JHW Zhou, Gerchman, Ramakrishnan, Travers, Muyldermans Nature 395, 402 (1998) An, Leuba, van Holde, Zlatanova PNAS 95, 3396 (1998) 3-99 Copyright 1999, J.H.Waterborg, UMKC H3 Chromatin fibers + charged N termini (bind DNA on neigboring nucleosomes) 11 nm (beads) highly acetylated core histones (especially H3 and H4) • HIGH level of histone H1 • Reduced level of histone H1 • NO gene transcription • Gene transcription possible JHW 3-99 Copyright 1999, J.H.Waterborg, UMKC 30 nm chromatin fiber Histone modifications 27 Turner (2002). Cell 111, 285-91 Acetylation of conserved lysines The N-termini of histones H4 and H3, and their acetylation patterns, are absolutely conserved. 5 Ac Ac Ac Ac 9 Ac 18 Ac 14 Ac Acetyl-CoA O 27 Ac or Me P O DNA P backbone binding Histone Deacetylase reversible reactions CoA O O C C g C e N+ O C a b C d C HAT (Histone Acetyl-Transferase) - - - - C C e C C C N C O no DNA binding - - - - - - - - - - P C C O - - N e-N-Acetyl-Lysine JHW 23 Ac A-R-T-K-Q-T-A-R-K-S-T-G-G-K-A-P-R-K-Q-L-A-T-K-A-A-R-K-S-A-P+ + + + + + + + + N Lysine 20 Ac or Me Ac-S-G-R-G-K-G-G-K-G-L-G-K-G-G-A-K-R-H-R-K-V-L-R-D+ + + + + + + + + + 4 Me H3 N-terminus 16 12 - - - - 3-99 Copyright 1999, J.H.Waterborg, UMKC H4 N-terminus 8 Gel Electrophoresis of Histones gel type: SDS AU _ electrode: size + charge + hydrophobicity size + charge Separation by: size AUT ++ + + H3.1 + TTTT 135 TT T T + + 34 H3.2 +1 2 ++ H3 + H3.1 0 135 H3.2 T TT T T 4 T T 3 4 3 2 1 0 2 H3 135 1 0 H3 H4 H4 H3 4 3 2 + + H4 + 4 3 TT 2 1 0 T 135 aa ++ H4 + 1 H4 0 102 H4 102 102 aa electrode: JHW + _ Gel scans: alfalfa histones (Waterborg, 90’s) _ 3-99 Copyright 1999, J.H.Waterborg, UMKC T Almost All Acetylation is Dynamic Chlamydomonas reinhardtii Level of histone acetylation: 32 % of H3 Average level of acetylation: 2.4 AcLys/H3 Average AcLys turnover T½: 1.9 0.4 min (H3) AcLys turnover T½ (min): 2.8 1.8 1.8 1.6 1.5 16 % of H4 1.6 AcLys/H4 3.5 1.1 min (H4) 25 % 20 25 protein Transcriptionally active fraction of genome 20 % Steady-state [*Ac] labeling 15 H3 H4 protein 20 15 10 10 5 5 0 5 4 LEVEL of AcLys turnover 0 LEVEL of AcLys turnover 5 4 3 3 2 2 1 1 0 0 1 2 3 4 5 1 2 % 3 4 5 #AcLys/histone Fraction of acetylated histone 100 92 104 106 82 39 58 81 100 76 % subject to AcLys turnover: 100 12 % of H3 55 4 % of H4 JHW Waterborg J. Biol. Chem. 273, 27602 (1998) (67% of multi-acetylated H4) 3-99 Copyright 1999, J.H.Waterborg, UMKC Example: alga Acetylation of Chromatin Domains Example: the chicken b-globin gene domain a(Ac-Lys) antibody nucleosome ppt DNA remaining domain boundary 10 30 kb 20 b bH bA be DNA loop domain domain boundary b = b-globin genes: DNase I hyper-sensitive site Control: inactive gene chicken ovalbumin General DNase I sensitivity DNase I l High levels of chromatin acetylation, across complete chromatin domains (DNA loops), induces chromatin changes detected as “general DNase I sensitivity” l Within these chromatin domains, at functional genes or transcription factors, the chromatin structure is interrupted by small “DNase I hypersensitive sites” JHW Hebbes, Clayton, Thorne, Crane-Robinson EMBO J. 13, 1823 (1994) 0 1 2 DNase I (U/ml) 3-99 Copyright 1999, J.H.Waterborg, UMKC 0 Acetylation at Promoters TR/RXR example of DNA-binding Transcription Factor TH ADA2 ADA3 GCN5 HAT HAT TAFII250 JHW p300 CPB HAT P/CAF Transcriptional REPRESSION Thyroid Hormone Receptor Without Thyroid Hormone co-activator co-repressor Histone Acetyl Transferases pol.II Adapted from Wolffe Nature 387, 16 (1997) Histone Deacetylase N-CoR Sin3 RPD3 HAT TAFII250 3-99 Copyright 1999, J.H.Waterborg, UMKC Transcriptional ACTIVATION + hormone TH Deacetylation of Chromatin Transcriptional Silencing — X-chromosome Inactivation 3’..pGpCp..5’ 5 me 5’..pCpGp..3’ MeCP2 transcriptional repressor co-repressor Sin3 Histone Deacetylase RPD3 + l 5meC CpG-methylation is maintained after DNA replication by Maintenance Methylase action on hemi-methylated DNA l 5meC binds transcriptional repressor MeCP2 (MethylC-binding Protein-2) l MeCP2 binds Sin3 with RPD3 histone deacetylase + CpG methylation JHW l 5meC CpG DNA modification is observed in repressed genes and inactivated X chromosomes Hypo-acetylated repressed chromatin fiber Nan et al. Mol.Cell.Biol. 16, 414 (1996); Cell 88, 1 (1997); Jones et al. Nat.Genet. 19, 187 (1998) 3-99 Copyright 1999, J.H.Waterborg, UMKC 5 me Histone Methylation Histones can be methylated at lysines or arginines. Example: H3 K9 methylation N Lysine S-adenosylmethyionine C C g C e N+ O C a b C d C HMT (Histone Methyl-Transferase) Histone demethylase? N e-N-monomethyl-Lysine C C C O C C e N+ C C S-adenosylmethyionine HMT (Histone Methyl-Transferase) N e-N-dimethyl-Lysine S-adenosylmethyionine C C C O C HMT (Histone Methyl-Transferase) C N e-N-trimethyl-Lysine C C O C C e N+ C C C C e N+ C C C C DNA backbone binding may not be strongly affected, but specific proteins may recognize these modifications Histone methylation : Histone methyltransferases Enhancer of Zeste & K27 Silencing of euchromatic genes Modified from: Lachner and Jenuwein (2002). Current Opin. Cell Biol. 14, 286-98 Multiple roles of H3 K9 methylation Tri-; di-; mono-HMTases? S. pombe to man (Tri-met K27) and tri- met K9 N. crassa Modified from: Lachner and Jenuwein (2002). Current Opin. Cell Biol. 14, 286-98 Enhancer of zeste In contrast to histone acetylation, histone methylation is STABLE. This makes of this mark a candidate for inheritance of chromatin states. Heterochromatin First discovered in Drosophila in genetic studies of chromosome rearrangements Heterochromatin induces gene silencing Singh, P. B. (1994). Molecular mechanisms of cellular determination: their relation to chromatin structure and parental imprinting. J Cell Sci 107, 2653-2668. Genes involved in heterochromatin Singh, P. B. (1994). Molecular mechanisms of cellular determination: their relation to chromatin structure and parental imprinting. J Cell Sci 107, 2653-2668. Heterochromatin formation involves histone H3 lysine 9 methylation by Su(var)3-9 and recruitment of HP-1 Ac Ac K9 K14 H3 Open chromatin HDAC K9 K14 H3 Methylation of K9 (Clr4; Suvar39) Me K9 K14 Recruitment of Swi6 or HP-1 Swi6 Me K9 H3 K14 H3 Recruitment of more Clr4 (Suvar39) molecules by Swi6 (HP-1) Condensed chromatin Epigenetic regulation of centromere function and RNAi Outer repeats Central domain Outer repeats S. Pombe Centromere dsRNAs Nucleosomes K9 Me Swi6 Nucleosomes K9 Me Swi6 This structure requires proteins that are responsible for the phenomenon of RNA interference -> RNA interference is gene silencing mediated by short RNA molecules produced from double stranded RNA (dsRNA). -> Short RNAs are produced by cleavage of dsRNA by the nuclease called Dicer. Dicer produces short dsRNA called siRNA duplexes. Then, a complex of proteins called RISC unwinds the duplex and produces siRNA. siRNA hybridize to the target mRNA which is degraded. …But RNAi also acts on chromatin -> Centromeres produce dsRNA, and Dicer as well as the RISC are required for centromere silencing. Transposons induce gene silencing in a similar manner. Mechanism for coupling of RNAi and chromatin regulation? Model: Nascent RNA si RNA K9 methylation, Swi 6 recruitment! Ago (RISC), Clr4 Dcr complex See also: Grewal and Moazed (2003). Science 301, 798-802 Opposing functions of H3 K9 versus K4 methylation Lachner and Jenuwein. (2002) Current Opin. Cell Biol. 14, 286-98 A functional link between histone H3 methylation and DNA cytosine methylation in Arabidopsis thaliana HP1? Modified from: Lachner and Jenuwein. (2002) Current Opin. Cell Biol. 14, 286-98 Multiple biological phenomena involving histone methylation E(z) H3-K27 methylation and and H3-K27 methylation Modified from: Lachner and Jenuwein. (2002) Current Opin. Cell Biol. 14, 286-98 Summary and perspectives Chromatin is packaged in a hierarchy of structures. Each of these levels of packaging has regulatory roles in the genome The level of packaging we know best, also thanks to formidable tools & technology development in the recent years, is the nucleosome. In particular, post-translational histone modifications play key roles in regulation of genome function, and the combinatorial power of these modifications is only beginning to be unraveled. Future challenges will be to decrypt the detailed meaning of these modifications, and to understand the roles of the higher order levels of chromatin and chromosome folding