pp02-DNA and Replication

DNA and Replication
Fig. 5-27
5
C
3
C
5
end
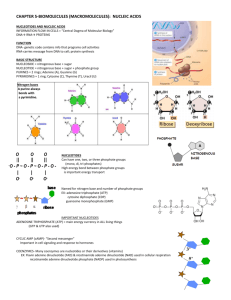
Making a nucleotide
Nucleoside
Nitrogenous base
Nitrogenous bases
Pyrimidines
Cytosine (C) Thymine (T, in DNA) Uracil (U, in RNA)
Purines
Phosphate group
5
C
3
C
3
end
(a) Polynucleotide, or nucleic acid
(b) Nucleotide
Sugar
(pentose)
Nitrogenous base connected to 1’ carbon of sugar – nucleoside
Phosphate group added to 5’ carbon of sugar - nucleotide
Adenine (A)
Sugars
Guanine (G)
Deoxyribose (in DNA)
(c) Nucleoside components
Ribose (in RNA)
3’ C
3’ C
(free)
Fig. 16.5
5’ C
(free)
5’ C
1’ C
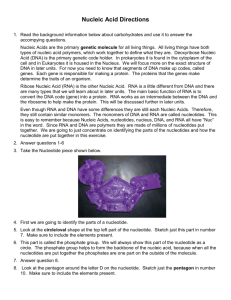
DNA is a linear polymer of nucleotide subunits joined together by phosphodiester bonds - covalent bonds between phosphate group at 5’ carbon and 3’ carbon of next nucleotide – uses oxygens as bridges.
Chain of nucleotides has alternating sugar and phosphate components, called the “sugarphosphate backbone.” Nitrogenous bases stick off backbone at regular intervals.
Note that any linear chain of nucleotides has a free 5’ C on one end, and a free 3’ C on the other. A chain of DNA thus has
POLARITY!
The polarity of this strand is
5’ -> 3’, top to bottom.
All strands of DNA look like this, there is no variability in the sugar phosphate backbone.
They differ in the identities of the nitrogenous bases at any given position – they have different DNA sequences. A simple way to represent this strand of DNA is:
5’-TACG-3’
Segments of this sequence, which can be
100s to 1000s of nucleotides long, are the genes that code for single, specific proteins.
free 3’ C
Fig. 16.5
5’ C
(free)
RNA
Sugar is ribose
Nitrogenous bases are A G C U
This is form of most RNAs in our cells.
DNA
Sugar is deoxyribose
Nitrogenous bases are A G C T
DNA takes structure one step further – almost always exists as a double helix.
Fig. 16.7
major groove minor groove
In a double helix, 2 strands of DNA wrapped around each other in shape of helix
Strands are held together by hydrogen bonding between nitrogenous bases. Weak interactions, but strength in numbers
Only pairings that work are A with T and G with C. Strands held at constant distance from one another because of the similar geometry of A-T and G-C base pairs
Also, only way pairings will work is if strands have opposite polarity
Fig. 16.8
The only pairings that work are A-T (2
H-bonds) and G-C (3 H-bonds). These are called nitrogenous base pairs (or simply base pairs).
Note that A-T and G-C base pairs both contain a purine and a pyrimidine – similar geometry, same overall diameter.
5’
3’ 5’
5’ 3’
The two strands of the DNA double helix are COMPLEMENTARY to each other. Means that when oriented with opposite polarity, their nitrogenous bases are in sequence to form perfect
Watson-Crick base pairs.
3’ 5’
3’
Watson and Crick suggested that to replicate DNA, strands were separated by breaking weak hydrogen bonds between base pairs. Each strand then has information to direct synthesis of new complementary strand to form 2 double helices.
DNA POLYMERASE
(in E. coli, three versions, I, II, and III)
Uses triphosphate forms of nucleotides as precursors.
Cannot initiate new strand, can only ADD nucleotides to 3’ end of growing strand that are complementary to template strand.
Synthesis ALWAYS in 5’ to 3’ direction.