PowerPoint 演示文稿
advertisement
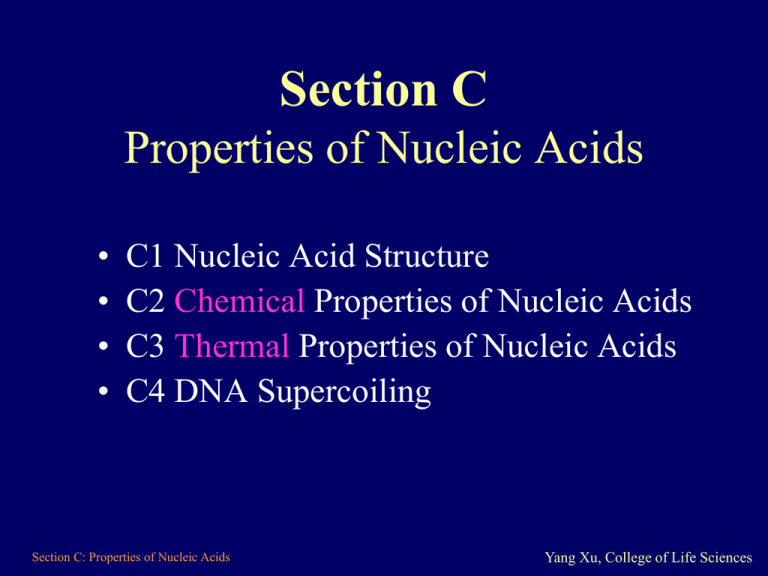
Section C Properties of Nucleic Acids • • • • C1 Nucleic Acid Structure C2 Chemical Properties of Nucleic Acids C3 Thermal Properties of Nucleic Acids C4 DNA Supercoiling Section C: Properties of Nucleic Acids Yang Xu, College of Life Sciences C1 Nucleic Acid Structure • • • • • Bases Nucleosides Nucleotides Phosphodiester DNA double helix Section C: Properties of Nucleic Acids Yang Xu, College of Life Sciences Bases • Purines are bicyclic structures, include: – Adenine and guanine • Pyrimidines are monocyclic, include: – Cytosine, thymine and uracil • T or U – The thymine base is replaced by uracil in RNA – Thymine is 5-methyl-uracil O Uracil (U) O Thymine (T) Section C: Properties of Nucleic Acids NH3 Aderine (A) NH NH33 O Guanine (G) Cytosine (C) Yang Xu, College of Life Sciences Nucleosides • Nucleosides = Base+Sugar, the bases are covalently attached to the 1'-position of a pentose sugar. Nucleotides 5 • Nucleotides = Base + Sugar + phosphates , in the 5'position of sugar, 1 - 3 phosphates may be attached. Phosphodiester bonds • Covalent linkage of a phosphate group between the 5'-hydroxyl of one ribose and the 3'-hydroxyl of the next. Section C: Properties of Nucleic Acids Yang Xu, College of Life Sciences DNA double helix • Discovery: In 1953 DNA double helix structure were deduced by Watson (34y) and Crick (46y) • Structure: – Two chains of DNA are in a righthanded double helix. – The sugar-phosphate backbones are on the outside, and the planar bases in the center of the helix. • Grooves: Between the backbone strands run the major and minor grooves. Section C: Properties of Nucleic Acids Yang Xu, College of Life Sciences Base pairing • Base pairs: The strands are joined by hydrogen bonding between the bases on opposite strands, to form base pairs. Section C: Properties of Nucleic Acids Yang Xu, College of Life Sciences C2 Chemical Properties of Nucleic Acids • Effect of acid • Effect of alkali Section C: Properties of Nucleic Acids Yang Xu, College of Life Sciences Effect of acid for DNA & RNA • Complete hydrolysis – In strong acid and at high temperatures, for example perchloric acid (HClO4) at more than 100 C, – nucleic acids are hydrolyzed completely to their constituents: bases, ribose (or deoxy-ribose) and phosphate. • Partly hydrolysis – In dilute acid, for example at pH 3-4, the most easily hydrolyzed bonds are selectively broken. – Apurinic hydrolysis: The glycosylic bonds attach the purine bases to the ribose backbone, if they are broken the nucleic acid becomes apurinic Section C: Properties of Nucleic Acids Yang Xu, College of Life Sciences Effect of alkali for DNA • DNA denaturation: The double-stranded structure of the DNA breaks down; that is the DNA becomes denatured. Effect of alkali for RNA • RNA hydrolysis: In alkali, the hydrolysis of RNA comes about, because of the presence of the 2‘-OH group in RNA, which is participated in the cleavage of the RNA back-bone by intra-molecular attack on the phosphodiester bond. Section C: Properties of Nucleic Acids Yang Xu, College of Life Sciences C3 Thermal Properties of Nucleic Acids • Thermal denaturation • Renaturation Section C: Properties of Nucleic Acids Yang Xu, College of Life Sciences Thermal denaturation • Increased temperature can cause the thermal denaturation of DNA and RNA: – RNA denatures gradually on heating, but – dsDNA ‘melts’ into single strands at a defined temperature, melting temperature (Tm), • Tm is a function of G+C content of the DNA • Denaturation may be detected by the change in A260. Section C: Properties of Nucleic Acids Yang Xu, College of Life Sciences Renaturation • The thermal denaturation of DNA may be reversed by cooling the solution. • The speed of cooling has an influence on the outcome: – Rapid cooling allows only to form dsDNA in local regions, it is not the original dsDNA molecule. – Slow cooling allows the sample fully double-stranded, with the same absorbance as the original dsDNA sample. • The renaturation between different nucleic acid strands is known as hybridization. Section C: Properties of Nucleic Acids Yang Xu, College of Life Sciences C4 DNA Supercoiling • • • • • • Closed-circular DNA Supercoiling Topoisomer Twist and writhe Energy of supercoiling Topoisomerases Section C: Properties of Nucleic Acids Yang Xu, College of Life Sciences Closed-circular DNA • Many DNA molecules in cells consist of closed-circular double-stranded molecules, for example: – bacterial plasmids; – bacterial chromosomes; – many viral DNA molecules. • This means that: – the two complementary single strands are each joined into circles, and has no free ends; – the molecules are twisted around one another and the two single strands are linked together a number of turns in the molecule. • This turn number is known as the linking number. Section C: Properties of Nucleic Acids Yang Xu, College of Life Sciences Supercoiling • Supercoiling: – It is the helix over the helix of dsDNA; – It happens in the closed-circular dsDNA. • Supercoiling direction: – Positive: the twist is in same direction as the double helix; – Negative: the twist is in opposite direction as the helix. • Lk and Lk – Lk : the value for a relaxed closed circle; – Lk: Lk = Lk - Lk, defined as the number of 360 twists introduced before ring closure. It quantifies the level of supercoiling. • Example: Most natural DNA is negatively supercoiled – DNA when isolated from cells is commonly negatively (-) supercoiled by around 6 turns per 100 turns, – that is Lk/Lk = -6/100 = -0.06. Section C: Properties of Nucleic Acids Yang Xu, College of Life Sciences Positive Negative Section C: Properties of Nucleic Acids Yang Xu, College of Life Sciences Topoisomer • The Lk is a topological property of a closed-circular DNA; • The linking number cannot be changed without breaking one or both of the DNA back-bones. • A molecule of a given linking number is known as a topoisomer. • Topoisomers differ from each other only in their linking number. Section C: Properties of Nucleic Acids Yang Xu, College of Life Sciences Twist and writhe • Topological changes The conformation of the DNA can be altered while the Lk remains constant (Fig. 2), corresponding to the types of the supercoiling (Lk), the DNA may be: – Completely into writhe (p45 Fig. 2a); – Completely into twist (p45 Fig. 2c); – Common situation is between the two extremes (2b). Section C: Properties of Nucleic Acids Yang Xu, College of Life Sciences Twist and writhe • Tw and Wr: – Tw: Twist linking number – Wr: Writhe linking number • Lk = Tw + Wr: – Lk must be an integer, but – Tw and Wr need not. Section C: Properties of Nucleic Acids Yang Xu, College of Life Sciences Energy of supercoiling • Torsional stress: – Supercoiling can introduce torsional stress into DNA molecules. Supercoiled DNA hence has a higher energy than relaxed DNA. • Roles of torsional stress: – For negative supercoiling, this energy makes it easier for the DNA helix to be locally untwisted. – Negative supercoiling may facilitate the processes which require unwinding of the helix, such as transcription initiation or replication. Section C: Properties of Nucleic Acids Yang Xu, College of Life Sciences Topoisomerases • Topoisomerases are essential enzymes in all organisms – Being involved in replication, recombination and transcription. • Topoisomerases: The enzymes that regulate the level of supercoiling of DNA molecules are termed topoisomerases – To alter Lk: they break transiently one or both DNA strands; – By attacking a tyrosine residue on a backbone; • There are two classes of topoisomerase: – Type I: breaking one strand of the DNA, and change the Lk in steps of ±1 (p46 Fig. 4a). – Type II: require the hydrolysis of ATP, break both strands of DNA and change Lk in steps of ±2 (p46 Fig. 4b). Section C: Properties of Nucleic Acids Yang Xu, College of Life Sciences Section C: Properties of Nucleic Acids Yang Xu, College of Life Sciences Segregation Section C: Properties of Nucleic Acids Yang Xu, College of Life Sciences That’s all for Section C Section C: Properties of Nucleic Acids Yang Xu, College of Life Sciences