physics
advertisement

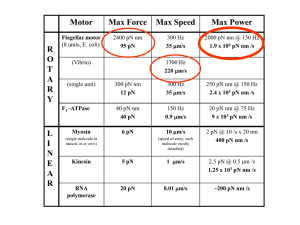
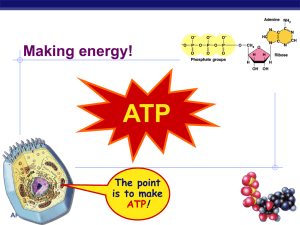
Welcome to BioPhysics 401 & Physics 475 Understanding biology using “simple” ideas from physics. with an emphasis on single-molecule biophysics You’ve (hopefully) made a good choice! Course Info: T, Th, 9:30 am-10:50 pm, 136 LLP Your host: Professor Paul Selvin; Office: 365 Loomis Office Hr: after class : 11-12:00pm on Tuesday. selvin@illinois.edu: 244-3371 (office) Your co-hosts: Marco Tjioe: Biophysics student (Taught this several years). tjioe2@gmail.com: 328 Loomis: 217-721-9047 Huseyin Tas: Biophysics student (Took course a last year). htas2@illinois.edu: 328 Loomis: 217-721-7311 TA Office Hrs: either Sunday & Monday 6-7 pm, Rm 32 LLP if HW is due Tues. or Tues. (rm 271 LLP) & Wed. (136 LLP) 6-7 pm if HW is due Th. HW due 1 week from date of assignment On Homework, work together! Turn in separate HWs. All Lecture Notes, assignments… physics.illinois.edu/courses/ Then Physics 475 Goals of course 1. Learn some basic molecular biology. DNA (PCR, sequencing) Proteins... can do everything! 2. Learn how to apply basic physics to biology. Mechanics, Electricity & Light, Statistical Mechanics (Example today – What planets are life possible on?) 3. Learn about/type problems biophysicists work on. Biology... Molecular motors (chemical mechanical) Ion Channels (chemical Ion Gradients electrical) Nerves (the physical underpinnings of memory) Photosynthesis (light electricalchemical energy) Stochastic Nature of gene expression, Magnetic Navigation, Vision... 4. Learn“back of the envelope”type calculations. Example today: Strength of animals 5. Learn experimental (bio)physics How to measure (nm distances, pN forces), Single molecules (Fluorescence, Optical & Magnetic Traps), Patch Clamp Techniques Some guest lecturers– people doing the stuff! Klaus Schulten, TJ Ha, Yann Chemla, Tom Kuhlman Course Information P475/ BP401 Introduction to Biological Physics You’ve (hopefully) made a good choice! Prerequisites Interest in getting a semi-quantitative view of biology using physical tools/idea. This is a diverse crowd, some bio-, some physicists, ½ undergrads, ½ grad. Minimal bio required. Do the reading & listen to lectures to: learn basics of bio, chem, stat mech. Physics 111, 112 (or equivalent), basic calculus Some knowledge of Statistical Mechanics. Will present new topics w old topics to keep it interesting. Ex: “Old” Topic: Central Dogma of Molecular Biology: DNA RNA Proteins. (Read this week’s homework.) Interesting (new) variant: (my lectures) DNA pre-mRNA mRNA Proteins (more later). Why little worm (19,735 genes) has as many genes as a human (~21k)! 1 mm____ Required Reading No one textbook, although web pages etc. At nih.gov, there are several books for free. http://www.ncbi.nlm.nih.gov/books/NBK21154/ (Can read but can’t browse) http://www.ncbi.nlm.nih.gov/books/NBK21054/?term=alb erts 1st homework: Read Chpt 1 of Berg et al.. Learn DG, (Free energy); DS, (Entropy); Types and strength of bonds Ready to put your thinking caps on? Typical calculations… Boltzman factor + Partition function (review of basic Stat. Mech. – see Kittel, Thermal Physic)s E1 Temp, T E0 If T = 0 ºK, what proportion of particles will be in E1, Eo? Answer: Eo= 1 E1 = 0 If T > 0 ºK, what proportion of particles will be in E1, Eo? P E i co n st. e ( )= P(E ) P E1 0 E i / kT e( ) - E1 -E0 kT Boltzman factor P E 1 i const. = 1 N e 1/ Z -E j / kT j= 0 Z = partition function N e j= 0 ( ) J= represents jth state 1 -Ei /kT P Ei = e z -E j / kT Partition Function for 2-state system State 0: Energy E0 State 1: Energy E1 ( ) P E1 = e e -E1 /kT -E o /kT +e -E1 /kT Simple case: Ball in gravitational field. Thermal fluctuations, finite probability of being at height, h. E = ?? E = m gh Eo h = 0 E 1 h = (m g )(h m eter) P (h ) P (0 ) = e -m g h / k T As ball gets smaller, probability gets smaller / larger ? “Ball” the size of O2? Why can you breathe standing up? What is 1/e height for O2? For O2, 1/e height is ~10 km ~height of Mt. Everest. (10 kM is “death zone”) Probability of dying if you go over 20,000 ft is 10% for every trip!! Grading (may be modified slightly if changes to course ) Grading 30%: written Homework : (about 9 total; drop lowest 1): (You CANNOT drop the last homework!) Work together, but turn in separately. Hand in at start of class– in class! (Do not be late.) 5% in-class (occasional) Quizzes: 20%: 10 pg. Written Report: (double-spaced, figures and references are in addition). 15% on midterm exam 20% on final exam 10% on classroom participation /class evaluation The Written Projects will be on a research paper you choose from the literature (ideally from Science, Nature, Cell). You will have to explain it for a literate, intelligent reader (like a reader of Scientific American). You will have to choose, in addition to the original research article, a general review article on the subject, and usually, a general textbook/web-site, must be cited/read by you. Plagiarism Not allowed! You will flunk the course. In written project… Something has always been written… (unless you have truly come up with something new.) Usually what’s written will be clearer than you can write it. But you want to independently understand it. And show me that you independently understand it. So… Read book/article, then close it, Then write your own version. This way you know you understand it, and can explain it in your own words. Also, when you “steal” a picture from somewhere, write in your paper where you got it from. A picture is worth a thousand words, but give credit where due. Yes, you get to evaluate class! Three (or 4) questions: 1. What was the most interesting thing you learned in class today? 2. What are you confused about? 3. Related to today’s subject, what would you like to know more about? 4. Any helpful comments. Answer, and turn in at the end of class. (I’ll give you ~5 minutes.) I’ll typically start class with some of your questions. Physics is about great laws Some examples… Newton’s three Laws (Mechanics) Isaac Newton, 1642-1727 Maxwell’s four Equations (Electricity & Magnetism) James Clerk Maxwell 1831-1879 Erwin Schrödinger 1887-1961 Schrodinger’s Eq’n (Quantum Mechanics) Does Biology have any great theories/laws? Charles Darwin, Age 51, 1860, On the Origin of Species Evolution -- Life evolved from simpler forms --One of the best tested scientific theories around Evolution is a series of tricks/random events Build complex beings from simpler parts Often many ways of doing this Our life form is just one. Difference between Physics, Biology and Biophysics Physics: understand inanimate (non-living) objects •Typically quantitatively (math) •E.g. how much do things attract •Experiment likely fitting into theory Biology: understand animate (living) objects •Typically qualitatively (math) •Things attract •Experiment Theory difficult Biophysics: understand animate (living) objects •Typically semi-qualitatively (math) •Order of magnitude estimation •(Emphasis will not be on math, but will be expected to know basic Calculus– see handout. •Experiment w Theory sometimes possible What is Life? (with a few sidetracks) Animate vs. Inanimate…Why your puppy is different than a rock, despite they’re both made of atoms which obey the same physical laws 4 things: A living system can: 1. Grow (but so can a lot of things, like a rock) 2. Consume energy from environment (but so can a lot of things, like a rock heating up) Earth/Us: All energy comes from the sun photons. Plants use photosynthesis to convert photons into chemical energy, namely ATP; animals eat plants (and other animals) Chemical energy of ATP ~ 2 photons of energy Small unit of energy Can you calculate this? How much energy in ATP? How much energy in a photon? What is Life? 4 characteristics Living things can 1) Grow, 2) Consume energy 3) Reproduce But there are exceptions to these: •A dog (or person) which is sterilized is considered to be still alive. • Mule (= offspring of a donkey and horse) can’t reproduce, but still is alive. • A Virus can’t reproduce without hijacking bacterial molecules. Is it alive? 4) Die You stop living..., but not much of a definition for living to say living is not to die. If you apply these ideas to species (a species is a group that can mate with others of its own group), things work out better. What is Life? (This slide is a sidetrack!) ATP Tri-P: lots of negative charge held tightly together F = kq1q2/r2 Adenine = recognition Ribose = handle Adenosine = Adenine + ribose recognition You should be able to calculate how much energy there is! Will have to take into account E, S (entropy) You eat sugar, proteins, etc., and make ATP. 1 glucose (sugar) = 36 molecules of ATP Process: called cellular respiration, largely done in the Mitochondria. Needs oxygen to be efficient: why we breathe in oxygen. Amount of energy for 1 molecule of ATP? (much less than sugar!) Usually express energy in units of kBT, Often in pN-nm: picoNewton - nanometers Recall that N-m = force x distance = Work or energy How much energy is in ATP (still sidetrack) 1 ATP = 20-25 kBT = 80-100 pN-nm 1 kBT = 4 pN-nm kB = 1.38 × 10-23 m2 kg s-2 K-1 (ouch!) kBT = the amount of energy that a “simple” molecule has at temp T (more later) Note: T in absolute temperature, i.e. Kelvin We often use pN-nm as units of energy. Energy = force x distance = newtons x meters If you prefer: 1 mole (6.0 x 1023 molecules = NA) where NA = Avogado’s number 1 mole of gas has 1RT of energy R= N x kB ATP = has RT of energy Imagine you have a little “car”, also called a molecular motor, which (naturally) uses ATP to do its work in a cell. Homework Read Chapter 1 by Berg On web-site under HW 1. Have it read by next Tuesday. Expect a 10 minute quiz covering main topics Thursday will assign Homework Set #1 On web-site under HW 1. (PDF is there.) Evaluate class 1. What was the most interesting thing you learned in class today? 2. What are you confused about? 3. Related to today’s subject, what would you like to know more about? 4. Any helpful comments. Put your name in upper right-corner. Then tear off your name before turning in. (That way you can be brutally honest!) Answer, and turn in at the end of class. (I’ll give you ~5 minutes.)