Adhesion molecule
advertisement

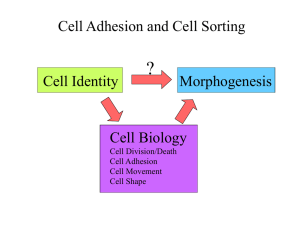
Cell adhesion Láng, Orsolya MD, PhD Dept. Genetics, Cell & Immunobiology, Semmelweis University Lecture EPh 2014 www.dgci.sote.hu Cell adhesion Contact with other cells and extracellular matrix Role: - embryonic development: formation of layers, tubes - connection and communication between cells - barrier, membrane polarity, mechanical attachment - cell motility - signal transduction - cancer progression Cell-cell adhesion molecules 1. Cadherins 2. CAMs or Ig like adhesion molecules Cell-ECM adhesion Molecules 4. Integrins 5. Proteoglycans (dystroglycan) 3. selectins Homophyl or heterophyl Ion dependency :Ca++, Mg++ Cytoskeletal component Junctional complex Cadherins Homophil connection Cell – cell Ca++ - dependent binding I.c. anchored to actin or intermedier filamentum Significant role in development of vertebrates Cell specificity N – neural P – placental E - epithelial Cadherin superfamily classical Non-cl Type Localisation Adherent E Epithelial Adherens junction N Neuronal, muscle, fibroblast Adherens junction Synapsism P Placenta, epidermis, mammary gland Adherens junction VE Endothelial Adherens junction Desmocollin Skin Desmosomes Desmoglein Skin Desmosomes T cadherin Neuronal, muscle - Cadherin 23 Inner ear Stereocilia Cadherins allow cells to sort themselves Ig-like adhesion molecules Homophil connection - typical Heterophil connection - rare 5 pcs. Ig-type domain Ca 2+ indep. adhesion Linked to actin filaments More than 20 variations Expressed in the critical phase of development Tissue-specificity: N-CAM - neuron L-CAM – liver V-CAM1- vascular Intracellular In melanoma – relation between ICAM-1 density and the metastatic activity of the tumor Selectin molecules Lectin type proteins, Carbohydrate specificity Tissue-specificity: E - epithelial L - lymphoid P - placenta L selectin: it has role in the initial phase of adhesion; in newborns the level of L sel. is low – the low number of inflammations (?!) Integrin molecules Heterophil connection Ca2+-dependent binding Focal contacts Its i.c. linker proteins are i.e.. talin, a-actinin, vinculin RGD sequ. is significant in ECM binding Partner molecules: fibronectin laminin collagen Deficiency (b) – the adhesion of leukocytes affected, results the increase of inflammations Type of integrins Integrin Ligand Localisation Mutation of α or ß subunit α5ß1 fibronectin ubiquitous Early death of the embryo, α6ß1 laminin ubiquitous Early death of the embryo, severe skin blistering α7ß1 laminin muscle Musclar dystrophy αLß2 ICAM leukocytes Impaired recruitment of leukocytes, LAD, recurrent infections αIIbß3 fibrinogen thrombocyte bleeding, no platelet aggregation, (osteoporosis) α6ß4 laminin Hemidesmosome epithelial cells severe skin blistering Role of adhesion molecule in extravasation of leukocytes Adhesion molecules www.cbrinstitute.org/.../media/image1.html Functional classification of cell junction Functional classification of cell junction Anchoring – mechanical junction Barrier formation - separation Channel like (gap junction) – communication Signal relaying junctions sinapsm immunological sinapsism transmembrane proteins Anchoring junctions Junctional complexes Tight junction Zonula adherens Desmosome Gap junction Interdigitation Hemidesmosome Tight junction(TJ) = zonula occludens Isolate parts of plasma membrane (apical and basolateral) Completely encircle polarized cells Look like honey comb Molecular structure of tight junction Claudins and occludins (membrane proteins) zip two membranes together Stabilized by spectrin Connected to spectrin by adapter proteins ZO1 and ZO2 Regulation of tight junctions Belts of proteins that close extracellular space between cells Prevent passage of water and water-soluble substances Account for electrical resistance across epithelia Leaky epithelia where there is need for some traffic Hormones Vasopressin Cytokines Lack of ATP causes “leak” Extravasating leukocytes open tight junctions Tight junctions separate components of the plasma membrane as well Mechanical juncion: zonula adherens - adhesion belt Adherent junctions Hold cells tightly together Confer mechanical strength Common in tissue that are subject to severe stress such as skin and cardiac muscle Molecular structure of zonula adherens Belt like junctions located just below tight junction Simple points of attachment, do not contain channels connecting the interiors of the two attached cells Adhesion molecule -cadherin (E) Linker proteins - α, b and catenin, vinculin Cytoskeletal component - microfilament (actin) Desmosome = macula adherens Adhesion molecule cadherins Cytoskeletal component Intermedier filaments (i.e. keratin) Cytoplasmatic plaque Components of desmosomes The expression pattern of DSGs and DSC is tissuespecific and may even vary within one tissue, like in the different layers of the epidermis Spinous layer desmosome Hemidesmosome – Cell-ECM junction Fixing of epithelial cells to the basal membrane Focal adhesion Adhesion molecule - integrins /Linker proteins – plectin, dystonin/ Cytoskeletal component – intermediate filament (keratin) Desmosomes and hemidesmosomes both link to intermediate filaments Gap junction – channel forming junction Communicating junction 100-1000 connexon protein: connexin homomeric-heteromeric combinations Gap junction TEM SEM In plant cells plasmodesmata perform many of the same functions as gap junctions Interdigitation Wave-like plasma membrane extensions + desmosomes Characteristic of epithelial cell nucleus Basal striation Increases the surface of the basal membraen for molecular transport Basal striation mitochondria Basement membrane ECM- Extracellular matrix • Mechanical • Influence on migration of the cells • Regulation of activity of molecules released • Co-receptors Main components of ECM Protein component Glycan Laminin (LN) LNs are cross-shaped proteins. All LN isoforms contain α, β1 and β2 chains that are connected by disulfide bonds (18 isoform of laminin- diverse in tissue) LN binds to membrane receptors (integrins) of the overlying cells. LN attaches cells to the basal lamina. LN contains binding sites for other components of the basal lamina: type IV collagen, heparin, Molecular Biology of the Cell (© Garland Science 2008) Collagens Provide strength of ECM maintains form of tissue Most abundant protein in vertebrates Found as bundles throughout ECM Structure: Rigid triple helix of 3 intertwined polypeptide chains Unusual aa compositionn (hydroxilation) Collagen fiber consists of numerous fibrils (molecules, polypeptides) Structure and synthesis of collagene ER/Golgi: Pro-a-chains are produced, hydroxylated and glycosylated at selected Lys and Pro residues. The lack of vitamin C prevents hydroxylation → impaired fibril formation (scurvy). Processed pro-peptides assemble into triplehelical pro-collagen. Golgi: Disulphide bonds form between the Nand C-termini of procollagen. After exocytosis, N- and C-termini are trimmed, allowing fibril assembly Fibrils (diam.10-300 nm) More than 15 types I., II., III., V., XI. – formation of fibrils IV., VII. – network IX., XII. – association of fibrils Collagen Assembly Matrix types produced by vertebrate cells Anchor C O L L A G E N E I II III Proteogly. Receptor Cells fibronectin ChS, DS ChS HS, Hep. fibroblast chondrocyte hepatocyte epithel integrin IV laminin HS, Hep. laminin rec. V VI fibronectin fibronectin HS, Hep. HS integrin resting fibrobl. integrin resting fibrobl. epithel, endothel, regenerating hepatocyte Abbr.: ChS – chondroitin sulfate; DS – dermatan sulfate; HS – heparan sulfate; Hep - heparin Fibroblast surrounded by collagen fibrils Osteogenesis imperfecta – clinical manifestation Tissue elasticity Elastin Elastic fibers permit long-range deformability and passive recoil. Elastic modulus is ~0.1 MPa. This function is crucial for arteries, lung, skin and other dynamic connective tissues that undergo cycles of extension and recoil. The major component of elastic fibers is the thread-like protein elastin Fibrillins provide an outer structure for amorphous, cross-linked elastin. During ageing, elastin is degraded and becomes inflexible. Tropoelastine→ cross link→ functional elastin Fibronectin Glycoproteins Fibronectin is a dimer of two identical 250 kDa subunits Alternative splicing of one gene produces ~20 human FNs RGD (Arg-Gly-Asp) component is recognised by integrines Mediate the connection between the ECM and the cell membrane. FN exists as a soluble form (plasma FN) and cellular FN. Plasma FN is predominantly produced in the liver. Cellular FN is deposited into the ECM by a cell-mediated process, FN binds a variety of other proteins like integrins, heparin, collagen, fibrin. TEM structure of basal lamina ~ basement membrane In epithelial membrane – prevent cancer cell invasion Kidney – serves as a filtration barrier Molecular Biology of the Cell (© Garland Science 2008) Molecular structure of basal lamina Transmembrane Molecular Biology of the Cell (© Garland Science 2008) Structure of Hyaluronan HA is a large, unbranched and negatively charged polymer of repeating (225K) disaccharides. it is synthesized freely at the plasma membrane by hyaluronan synthases. Free HA is found in the ECM of migrating cells. HA binds to cell surface receptors (CD44) HA binds proteoglycans. Structure of aggrecan aggregate In cartilage the key proteoglycan is aggrecan (MW: 2 x 108) At 40 nm intervals aggrecan core proteins are attached (assisted by a linker protein) to a decasaccharide sequence in hyaluronan Attached to the aggrecan core protein are multiple GAGs The major GAGs in aggrecan are chondroitin sulphate and keratin sulphate Aggrecan Rheumatoid artritis Synovial fluid Serum Fimbrin Nexilin Molecular structure of the focal contact Tensin α-Actinin Talin Paxillin Caveolin Zyxin Palladin Vinexin Ponsin Integrin, Syndecan-4, Leukocyte common antigen Function of the adhesion molecules Adhesion molecule Summary I. Summary II. Molecular Biology of the Cell (© Garland Science 2008)