gg - Shippensburg University
advertisement

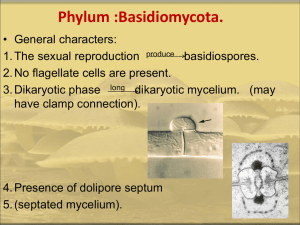
Ustilaginomycetes, The Smut fungi Mycology (Bio 594, Special Topics) M. Marshall, 2013 Shippensburg University (See last slide for additional credits) Agaricomycotina Mushrooms, puffballs, shelf fungi, jelly fungi Ustilaginomycotina Smut fungi and allied taxa Pucciniomycotina Rust fungi and allied taxa Begerow et al. 2006. Mycologia 98: 906-916 Subphylum Ustilaginomycotina • 1500 species, 80 genera • Mostly plant pathogenic fungi (biotrophs) – Infect > 4000 species of plants in 75 families of angiosperms • Group united by: – Sequence analysis of large subunit rDNA – Host/parasite zone of interaction – Septal pores lacking parenthesomes, but some with dolipore – Parasitic dikaryotic phase and saprotrophic haploid phase • Many taxa dimorphic, with yeast-like haploid phase and mycelial dikaryotic phase Interaction zones and septal pores in Ustilaginomycetes. From Bauer et al. 2001. The Mycota VII. Part B. Chapter 3. • Ustilaginomycetes – Ustilaginales – Urocystales • Exobasidiomycetes (we won’t cover) – – – – – – – Tilletiales Malasseziales Exobasidiales Georgefischeriales Entylomatales Doassansiales Microstomatales • Entorrhizomycetes – Entorrhizales The Smut Fungi • “Smut” term comes from the dark masses of teliospores formed by many members of the group Smut Fungi • Economically important pathogens include: – Ustilago maydis (corn smut) – Tilletia controversa (dwarf bunt of wheat) – Tillieta tritici and T. laevis (common bunt) – Tilletia indica (Karnal bunt of wheat) – Urocystis agropyri (flag smut) From Casselton and Olesnicky, 1998 Mating Systems in Smut Fungi • Heterothallic/tetrapolar (e.g., Ustilago maydis) – Two unlinked mating type (MAT) loci a and b • a locus – 2 alleles: a1 and a2 – Includes pheromone and pheromone receptor genes that control cell-to-cell signaling – controls fusion of haploid cells to form dikaryon • b locus – multiple alleles (25 in U. maydis) – regulates filamentous growth of dikaryon – is a main determinant of pathogenicity • Heterothallic/bipolar (e.g., Ustilago hordei) – a and b loci are physically linked on one chromosome Teliospore germination Basidiospores Basidium Ustilago-type Tilletia-type Basidial types in. From Bauer et al. 2001. The Mycota VII. Part B. Chapter 3. Ustilaginomycetes Dikaryon formation • Conjugation of primary or secondary basidiospores • Conjugation of basidium cells H-body Secondary Basidiospores (Tilletia) Forcibly discharged, formed from sterigma-like structure Passively dispersed Sorus (pl. sori) • Teliospores are formed in sori • Composed of host and fungal tissues • Formed in host ovaries, stems, leaves, or roots depending on the smut taxon • Characters of taxonomic importance include: – – – – Thread-like structures (fungal) Sterile cells Columella (host) Peridium (host or fungus) • Persistent = covered smut • Thin, breaking down to expose spores = loose smut Teliospores • Formed singly or in spore balls • Mostly globose, pigmented, with thick, ornamented walls • Size ranges from 3.5 to 60 µm diam • Resistant structures, in some species can survive up to 10 years in soil, and 25 years or more under optimal conditions Teliospores Smut Diseases • Based on location of sorus in host: – – – – Inflorescence smuts Leaf smuts Stem smuts Root smuts Common types of smut diseases • Bunt – Ovary-infecting species of Tilletia that infect cereals • Stinking Bunt – Diseases caused by Tilletia species that produce foetid (fishy) odor Common types of smut diseases • Partial Bunt – Only a portion of seed or inflorescences are bunted, only part of seed is replaced by sorus Tilletia walkeri infecting Lolium multiflorum Karnal bunt (Tilletia indica) Ustilago • Sori in reproductive organs or vegetative tissues of host • Teliospores formed singly, usually pigmented with sculptured walls • Sterile cells absent • Ustilago-type germination Common types of smut diseases • Covered smut – Well-developed, persistent peridium surrounding sorus • Loose smut – Thin, delicate peridium that ruptures easily to expose teliospores Tilletia • Sori usually in reproductive organs of host • Teliospores formed singly, usually pigmented with ornamented walls • Sterile cells present in sorus • Teliospores with foetid odor due to production of trimethylamine • Tilletia-type germination Corn Smut, Ustilago maydis Loose Smut of Wheat, Ustilago nuda, and U. tritici Covered Smut of wheat, Telletia carries, T. foetida. Smut “groups”- Overall Chart This outline is just provided as a convenience for study purposes - Obtained from Pl. Path. Dept., U. of Nebraska Smut groups sheet A Smut Groups sheet B Ignore the remainder Entyloma • Sori in vegetative organs of host • Teliospores formed singly, permanently embedded in host tissue • Teliospores with pale, smooth walls • Tilletia-type germination Urocystis • Sori mostly in leaves, stems, forming streaks, swellings or galls • Spore balls with pigmented teliospores surrounded by hyaline sterile cells • Tilletia-type germination Thecaphora • Sori in various parts of host, mostly reproductive organs • Spore balls comprising several to many, pigmented, wedge-shaped teliospores with sculptured outer walls • Germination by formation of septate metabasidium, cells form hyphae that fuse to establish dikaryon http://www.redepapa.org/thecaphora.jpg Order Exobasidiales • Plant parasitic fungi • Form holobasidia on leaves, no teliospores formed • Basidiospores become septate during germination • Dimorphic • Four families, two will be covered: – Exobasidiaceae – Graphioloceae Exobasidiaceae: Exobasidium Graphiola Credits This presentation has been modified from one posted on the web by Dr. Lori Carris, Washigton State University Plant Pathology Dept. from her course: Plant Path 521, Mycology.