Pentose Phosphate Shunt
advertisement

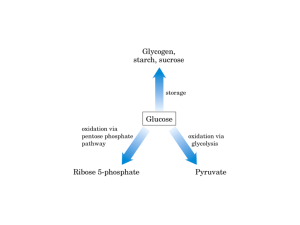
The Pentose Phosphate Shunt (AKA: Pentose Phosphate Pathway, PPP) Uses Glucose 6P to produce 3, 4, 5, 6 and 7 carbon sugars. In the process of doing this it reduces NAD+ to NADPH which is needed for reductive biosynthesis. NADPH is needed for synthesis of fatty acids Fatty acids are a highly reduced energy store Palmitate is the primary fatty acid, made to 16Cs by the serial addition of 2Cs from Acetyl-S-CoA Acetyl-S-CoA Just attaching Acetyls together would yield this, which is NOT found This is palmitate the primary product of the following reaction Fatty acid synthesis and NADPH continued 8Acetyl-CoA +7ATP +7H+ + H20 + 14 NADPH a 8 CoA + 7Pi +7ADP +Palmitate + 14 NADP+ So Palmitate and all other reduced fatty acids require the oxidation of NADPH to NADP+. Oxidation is the opposite of reduction. If something is reduced then something else must be oxidized. NADP+ is reduced to NADPH in the pentose phosphate pathway In NADP+ (oxidized) In NADPH (reduced) This phosphate is not present in NAD+ or NADH NADPH NH2 derived from niacin (vitamin B3) Dietary Deficiency of Niacin - Pellegra Disease characterized by the “three Ds” - dematitis, diarrhea, and dementia. “Pellegra” - Italian for “rough skin”. Mental hospitals in the southern states filled up every late winter/early spring with patients with pellegra. In 1930 there were about 200,000 cases of pellegra in the US. A man with an early stage of pellagra known as “pellagra gloves”. Pellegra or “corn disease” was originally thought to be an infectious disease or result from eating contaminated corn. 80% of niacin in corn is bound to a substance making it unavailable. Native Americans did not get pellegra because they treated their corn with alkali (lime or wood ashes) to make hominy before grinding it. Dr. Joseph Goldberger - realized pellagra was due to a dietary deficiency and was not an infectious disease. Figure 22.22 The pentose phosphate pathway. The Oxidative Steps of the Pentose Phosphate Pathway 1. Glucose-6-P Dehydrogenase Irreversible 1st step - highly regulated! 2. Gluconolactonase The uncatalyzed reaction happens too 3. 6-Phosphogluconate Dehydrogenase An oxidative decarboxylation (in that order) The Oxidative Steps of the Pentose Phosphate Pathway Figure 22.23 The glucose-6-phosphate dehydrogenase reaction is the committed step in the pentose phosphate pathway. Figure 22.24 The Gluconolactonase Reaction Figure 22.24 The gluconolactonase reaction. The uncatalyzed reaction also occurs. The Oxidative Steps of the Pentose Phosphate Pathway Figure 22.25 The 6-phosphogluconate dehydrogenase reaction. The initial NADP+-dependent dehydrogenation yields a β-keto acid, 3-keto-6-phosphogluconate, which is very susceptible to decarboxyation (the second step). The resulting product, D-ribulose-5-P, is the substrate for the nonoxidative reactions of the pentose phosphate pathway. Figure 22.22 The pentose phosphate pathway. The Nonoxidative Steps of the Pentose Phosphate Pathway Five steps, but only 4 types of reaction... Phosphopentose isomerase converts ketose to aldose Phosphopentose epimerase epimerizes at C-3 Transketolase ( a TPP-dependent reaction) transfer of two-carbon units Transaldolase (uses a Schiff base mechanism) transfers a three-carbon unit Figure 22.22 The pentose phosphate pathway. The Nonoxidative Steps of the Pentose Phosphate Pathway Figure 22.26 The phosphopentose isomerase reaction converts a ketose to an aldose. The reaction involves an enediol intermediate. Figure 22.22 The pentose phosphate pathway. The Nonoxidative Steps of the Pentose Phosphate Pathway Figure 22.27 The phosphopentose epimerase reaction interconverts ribulose-5-P and xylulose-5-phosphate. The mechanism involves an enediol intermediate and occurs with inversion at C-3. Figure 22.22 The pentose phosphate pathway. The Nonoxidative Steps of the Pentose Phosphate Pathway Figure 22.28 The transketolase reaction of step 6 in the pentose phosphate pathway. This is a two-carbon transfer reaction that requires thiamine pyrophosphate as a coenzyme. TPP chemistry was discussed in Chapter 19 (see the pyruvate dehydrogenase reaction). Figure 22.22 The pentose phosphate pathway. The Nonoxidative Steps of the Pentose Phosphate Pathway Figure 22.31 The transaldolase reaction. In this reaction, a 3-carbon unit is transferred, first to an active site lysine, and then to the acceptor molecule. Figure 22.22 The pentose phosphate pathway. The Nonoxidative Steps of the Pentose Phosphate Pathway Figure 22.29 The transketolase reaction of step 8 in the pentose phosphate pathway. This is another two-carbon transfer, and it also requires TPP as a coenzyme. Utilization of Glucose-6-P Depends on the Cell’s Need for ATP, NADPH, and Rib-5-P Glucose can be a substrate either for glycolysis or for the pentose phosphate pathway The choice depends on the relative needs of the cell for biosynthesis and for energy from metabolism ATP can be made if G-6-P is sent to glycolysis Or, if NADPH or ribose-5-P are needed for biosynthesis, G-6-P can be directed to the pentose phosphate pathway Depending on these relative needs, the reactions of glycolysis and the pentose phosphate pathway can be combined in four principal ways Four Ways to Combine the Reactions of Glycolysis and Pentose Phosphate 1) Both Ribose-5-P and NADPH are needed by the cell In this case, the first four reactions of the pentose phosphate pathway predominate NADPH is produced and ribose-5-P is the principal product of carbon metabolism 2) More Ribose-5-P than NADPH is needed by the cell Synthesis of ribose-5-P can be accomplished without making NADPH, by bypassing the oxidative reactions of the pentose phosphate pathway Four Ways to Combine the Reactions of Glycolysis and Pentose Phosphate Case 1: Both ribose-5-P and NADPH are needed Figure 22.33 When biosynthetic demands dictate, the first four reactions of the pentose phosphate pathway predominate and the principal products are ribose-5-P and NADPH. Four Ways to Combine the Reactions of Glycolysis and Pentose Phosphate 3) More NADPH than ribose-5-P is needed by the cell This can be accomplished if ribose-5-P produced in the pentose phosphate pathway is recycled to produce glycolytic intermediates 4) Both NADPH and ATP are needed by the cell, but ribose-5-P is not This can be done by recycling ribose-5-P, as in case 3 above, if fructose-6-P and glyceraldehyde-3-P made in this way proceed through glycolysis to produce ATP and pyruvate, and pyruvate continues through the TCA cycle to make more ATP Xylulose-5-Phosphate is a Metabolic Regulator In addition to its role in the pentose phosphate pathway, xylulose-5-P is also a signaling molecule When blood glucose rises, glycolysis and the pentose phosphate pathways are activated in the liver The latter pathway makes xylulose-5-P, which stimulates protein phosphatase 2A (PP2A) PP2A dephosphorylates PFK-2/F-2,6-Bpase and also carbohydrate-responsive element-binding protein (ChREBP) Glycolysis and lipid biosynthesis are both activated as a result Xylulose-5-Phosphate is a Metabolic Regulator Activation of PP2A triggers dephosphorylation of PFK-2/F2,6BPase, which raises F-2,6-BP levels, activating glycolysis and inhibiting gluconeogenesis. Figure 22.34 Dephosphorylation of ChREBP elevates expression of genes for lipogenesis. Aldose Reductase and Diabetic Cataract Formation The complications of diabetes include a high propensity for cataract formation in later life Hyperglycemia is the cause, but why? Evidence points to the polyol pathway, in which glucose and other simple sugars are reduced in NADPH-dependent reactions Glucose is reduced by aldose reductase to sorbitol, which accumulates in lens fiber cells, increasing pressure and eventually rupturing the cells Aldose reductase inhibitors such as tolrestat and epalrestat suppress cataract formation Aldose Reductase and Diabetic Cataract Formation Glucose is reduced by aldose reductase to sorbitol, which accumulates in lens fiber cells, increasing pressure and eventually rupturing the cells Aldose reductase inhibitors such as tolrestat and epalrestat suppress cataract formation Glucose 6-Phosphate Dehydrogenase Deficiency - affects more than 400 million people in the world. - most common enzyme abnormality (enzymopathy) in people. - the disease results in acute hemolytic anemia. -red blood cells are particularly affected because they lack mitochondria. -NADPH is important for maintaining adequate levels of reduced glutathione (GSH) in the cell. - The GSH is critical for destroying hydrogen peroxide and maintaining the cysteine residues in hemoglobin and other rbc proteins in the reduced state as well as the iron in hemoglobin in the ferrous state. Glucose 6-Phosphate Dehydrogenase Deficiency (cont’d) More than 400 variants of G6PD deficiency . Most of the mutations are single-base changes that result in an amino acid substitution. The G6PD gene is on the X chromosome so males are primarily affected. Most female carriers (heterozygotes) are asymptomatic. G-6-PD deficiency affects all races. The highest prevalence is among persons of African, Asian, or Mediterranean descent. Severity varies significantly between racial groups because of different variants of the enzyme. Glucose 6-Phosphate Dehydrogenase Deficiency (cont’d) About 11% of African-American males are affected by the so-called A-type. In Africa, female carriers of the mutation were found to have an increased resistance to malaria. People originating in the Mediterranean region may have another, more serious variant, called the Mediterranean type. People are usually asymptomatic but certain drugs can trigger a severe episode of hemolytic anemia within hours of exposure. Drugs that can bring on an acute reaction include: • • • • • • • • antimalarial agents sulfonamides (antibiotic) aspirin nonsteroidal anti-inflammatory drugs nitrofurantoin quinidine quinine others Bacterial and viral infections can also trigger episodes.