Chapter 3 Elasticity and Strength of Materials
advertisement

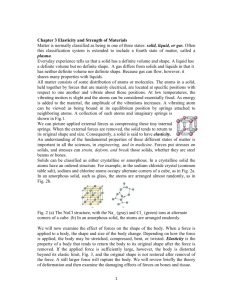
Chapter 3 Elasticity and Strength of Materials References 1-Physics in biology and Medicine 3rd e, Paul Davidovits 2- web sites 3- College Physics, 7th e, Serway April 13, 2015 1 Classification of matter • Matter is normally classified as being in one of three states: • A solid has a definite volume and shape. • A liquid has a definite volume but no definite shape. • A gas it has neither definite volume nor definite shape. Because gas can flow, however, it shares many properties with liquids. • Often this classification system is extended to include a fourth state of matter, called a plasma. April 13, 2015 2 Structure of matter • All matter consists of some distribution of atoms or molecules. • In a solid: The atoms, held together by forces that are mainly electrical, are located at specific positions with respect to one another and vibrate about those positions. • At low temperatures • The vibrating motion is slight and the atoms can be considered essentially fixed. • As energy is added to the material, • The amplitude of the vibrations increases. A vibrating atom can be viewed as being bound in its equilibrium position by springs attached to neighboring atoms. A collection of such atoms and imaginary springs is shown in Fig.1. April 13, 2015 3 Structure of matter • We can picture applied external forces as compressing these tiny internal springs. • When the external forces are removed, the solid tends to return to its original shape and size. Consequently, a solid is said to have elasticity. • An understanding of the fundamental properties of these different states of matter is important in all the sciences, in engineering, and in medicine. • Forces put stresses on solids, and stresses can strain, deform, and break those solids, whether they are steel beams or bones. April 13, 2015 4 Solid Classification • Solids can be classified as either: • crystalline: NaCl, • or amorphous: Glass April 13, 2015 5 Stress- Strain • • • • • • Examine the effect of forces on a body 1-stretched, compressed, bent, Twisted Elasticity is the property of a body that tends to return the body to its original shape after the force is removed. April 13, 2015 6 Longitudinal Stretch and Compression • Stress, S • Longitudinal Strain, St • Hook`s Law April 13, 2015 7 April 13, 2015 8 A Spring energy E stored in the spring is given by April 13, 2015 9 Fatigue • Fatigue is the progressive and localized structural damage that occurs when a material is subjected to cyclic loading. • Fatigue life, Nf, is the number of stress cycles of a specified character that a specimen sustains before failure of a specified nature occurs. • Surface fatigue: Surface fatigue is a process by which the surface of a material is weakened by cyclic loading. • Fatigue wear is produced when the wear particles are detached by cyclic crack growth of microcracks on the surface. These microcracks are either superficial cracks or subsurface cracks. April 13, 2015 10 Bone Fracture: Energy Considerations • Knowledge of the maximum energy that parts of the body can safely absorb allows us to estimate the possibility of injury under various circumstances. • Assume that the bone remains elastic until fracture, the corresponding force is April 13, 2015 11 Example • A leg bone 90 cm and an average • area of about 6 cm2 • Y=14×1010 dyn/cm2 • This is the amount of energy in the impact of a 70-kg person jumping from a height of 56 cm (1.8 ft), given by the product mgh. • • E= 70x10xH=384 J H=384/700=0.56 m= 0.56 cm April 13, 2015 12 Impulsive Forces • In a sudden collision, a large force is exerted for a short period of time on the colliding object. • For example, if the duration of a collision is 6×10−3 sec and the • change in momentum is 2 kg m/sec, the average force that acted during the collision is April 13, 2015 13 Fracture Due to a fall: Impulsive Force Considerations • The magnitude of the force that causes the damage is computed • the duration of the collision Dt is difficult to determine precisely • If the colliding objects are hard, very short~ few milliseconds • If the objects is soft and yields during the collision, the duration of the collision is lengthened, and as a result the impulsive force is reduced. April 13, 2015 14 Example • When a person falls from a height h, his/her velocity on impact with the ground, neglecting air friction • W=mg • After the impact the body is at rest : mvf = 0 • Measuring time is a problem • Vertical fall Dt=10-2 sec • bends his/her knees or falls on a soft surface April 13, 2015 15 • Table 3.1, the force per unit area that may cause a bone fracture is 109 dyn/cm2 • person falls flat on his/her heels, the area of impact may be about 2 cm2. Body of mass of 70 kg, Dt = 10−2 sec April 13, 2015 16 Airbags: Inflating Collision Protection Devices • The impact force may also be calculated from the distance the center of mass of the body travels during the collision under the action of the impulsive force. 30 cm v Decelerating force, F April 13, 2015 17 For A =1000 cm2 • At an impact velocity of 70 km/h • F= 4.45×106 dyn • Stress= 4.45×103 dyn/cm2 < The estimated strength of body tissue. • At a 105-km • F= 1010 dyn • Stress= 107 dyn/cm2. probably injure the passenger April 13, 2015 18 Whiplash Injury • the impact is sudden, as in a rear-end collision, • the body is accelerated in the forward direction by the back of the seat, the unsupported neck is then suddenly yanked back at full speed. April 13, 2015 19 Falling from Great Height • • • • • Falling on a hard surface Cause injury Energy=mgh=1/2 mv2 Falling on a soft surface Example: decelerating impact force acts over a distance of about 1 m, the average value of this force remains below the magnitude for serious injury even at the terminal falling velocity of 62.5 m/sec (140 mph). April 13, 2015 20 Nonomaterial • Nanotechnology • is the production of functional materials and structures in the range of 0.1 to 100 nanometers physical or chemical methods • one hydrogen atom is 0.1 to 0.2 nm and of a small bacterium about 1,000 nm • Nanotechnologies are predicted to revolutionize: • (a) the control over materials properties at ultrafine scales; and • (b) the sensitivity of tools and devices applied in various scientific and technological fields. April 13, 2015 21 Nanomaterials • It studies materials with morphological features on the nanoscale, and especially those that have special properties stemming from their nanoscale dimensions. • A bulk material should have constant physical properties regardless of its size, • At the nanoscale this is often not the case. Sizedependent properties are observed such as quantum confinement in semiconductor particles, and superparamagnetism in magnetic materials, etc.. April 13, 2015 22 Example • For example, • the bending of bulk copper (wire, ribbon, etc.) occurs with movement of copper atoms/clusters at about the 50 nm scale. • Copper nanoparticles smaller than 50 nm are considered super hard materials that do not exhibit the same malleability and ductility as bulk copper. April 13, 2015 23 Some recent publication in dentistry material science April 13, 2015 24 April 13, 2015 • Figure 1. Schematics and transmission electron microscopic images of composites studied. A. Composite with nanometric particles (× 60,000 magnification). B. Composite with nanocluster particles (×300,000 magnification). C. Composite with hybrid fillers (×300,000 magnification). • nm: Nanometers. APS: Average particle size. μm: 25 micrometer Assignment • Solve the following problems • 1, 3, 5 April 13, 2015 26