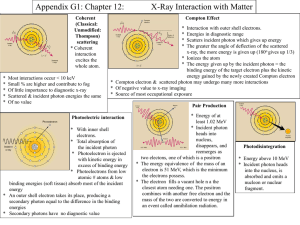
Thiel - Davidson Physics
advertisement
Investigating Electron Binding Energies of Impurity Ion States and Host Crystal Bands in Rare-Earth-Doped Optical Materials C. W. Thiela,b and R. L. Conea a Physics Department, Montana State University, Bozeman, MT, USA 59717 b Spectrum Lab, Montana State University, Bozeman, MT, USA 59717 Research was supported in part by the Air Force Office of Scientific Research, Scientific Materials Corporation, and the National Science Foundation Email: thiel@physics.montana.edu 17th International Conference on Dynamical Processes in Excited States of Solids, Argonne National Laboratory, June 2010 Electronic Structure of Rare-earth Materials Rare-earth Ion Host Crystal Conduction Band States 4f N15d Valence Band States 4f N The atomic-like electronic structure of localized rare-earth ion states is well understood The electronic band structure of de-localized crystal states is well understood To predict and explain many optical properties and electron transfer processes, it is essential to understand how these two classes of states are related and interact in rare-earth-activated optical materials 17th International Conference on Dynamical Processes in Excited States of Solids, Argonne National Laboratory, June 2010 Importance of Crystal Band States Broad Impact on Rare-Earth-Activated Optical Materials: Optical Memories and Processors—Photorefractive and photon-gated hole burning techniques may use photoionization for non-volatile operation Laser Materials—Excited-state absorption to conduction band can limit gain and tuning range and cause optical damage Phosphors and Solid-State Lighting—Ionization provides a non-radiative relaxation pathway while charge transfer provides an optical pumping mechanism Scintillators— Ionization reduces light yield while efficiency of energy transfer from electron hole pairs is influenced by relative energies of ion and band states Electroluminescence—Field-induced ionization and thermal ionization may limit performance in rare-earth-doped semiconductor materials Studying the Relationships Between Rare Earth and Band States: Need a broad picture for the electronic structure of the host-impurity system to understand optical properties of materials Motivates fundamental theoretical understanding Helps explain and predict optical properties of materials Guides the logical design of new materials with optimum properties 17th International Conference on Dynamical Processes in Excited States of Solids, Argonne National Laboratory, June 2010 Methods for Studying Electron Transfer There are many Methods for Probing Broad Energy Level Structure Optical Spectroscopy—Absorption or reflectivity spectra reveal charge transfer and photoionization transition energies, as well as fundamental host absorption Electron Spectroscopy—Photoemission and inverse photoemission directly measure electron binding energies of occupied and unoccupied electronic states Photoconductivity and Photocapacitance—Electron transfer detected from mobility of generated electron or hole charge carriers Thermally Stimulated Luminescence Excitation—Electron transfer detected in fluorescing materials from charge recombination and relaxation Microwave-detected Electron Transfer—Electron transfer detected by transient changes in the material’s dielectric constants and the effect on a resonant microwave cavity Photo-EPR—Electron transfer detected by change in ground state spin of ionized or reduced centers in the material, or EPR signature of trapped charges ...and others Each method has unique advantages and disadvantages, and generally a combination of methods is required to fully investigate the electronic structure 17th International Conference on Dynamical Processes in Excited States of Solids, Argonne National Laboratory, June 2010 Electron Photoemission Spectroscopy Incident photons eject electrons from the occupied states in the material Difference between Photon Energy (hnp) and ejected electrons’ Kinetic Energy (KE) gives the Binding Energy (BE) of the electrons in the sample The energy distribution of photoelectrons gives the binding energies of all occupied electronic states—provides relative energies of the 4f electrons and the host valence band KE Spectrum EVacuum Photoemission Directly Measures Electron Binding Energies Relative to a Common Energy Reference Host Conduction Band (CB) Extract Host and Ion Features: VBM Binding Energy Valence Band Maximum (VBM) hnp 4f Binding Energy RE3+ (4fN) Ground State Host Valence Band (VB) Resonant Photoemission (RPES) exploits resonances in the rare-earth PES crosssections to identify and extract 4f electron PES May also compare spectra of samples with different rareearth ion concentration 17th International Conference on Dynamical Processes in Excited States of Solids, Argonne National Laboratory, June 2010 Photoemission Final-state Structure The 4f photoemission exhibits structure extending over a range of up to 10 eV that corresponds to the tetravalent rare-earth ion final electronic states 30% Er:YAG We are interested in threshold energies—a method is required to estimate the minimum energy required to remove a 4f electron from the trivalent ion 4f Spectrum Final-state Structure Fit of FSS Photoelectron Counts The 4f photoemission “Final-state Structure” may be predicted from the electronic states of the tetravalent ions— related to “Coefficients of Fractional Parentage” The theoretical final-state structure is fit to the observed photoemission to accurately determine 4f binding energies 24 22 20 18 16 14 12 10 Electron Binding Energy (eV) 8 6 4 This final-state projection theory describes general trends in free-ion inter-configurational transition probabilities (4f-5d) 17th International Conference on Dynamical Processes in Excited States of Solids, Argonne National Laboratory, June 2010 Locating the Valence Band Maximum We are interested in comparing the energies of the 4f electrons to the energies of host band states—an estimate for the valence band maximum (VBM) is required for each material We estimate valence band photoemission cross-sections from theoretical atom-resolved partial density of states (PDOS) and atomic cross-sections Photoelectron Counts YAG hn = 125 eV PES Data Theory Fit of Theory Fit theoretical cross-section to spectrum to locate VBM The top of the valence band is very flat throughout the Brillouin zone for rare-earth oxides and fluorides VBM at 8.7 eV 20.0 17.5 15.0 12.5 10.0 7.5 Electron Binding Energy (eV) Orbital PES Cross Sections from Yeh & Lindau 1985 YAG PDOS from Xu & Ching 1999 5.0 Other approximations for VB structure may be used if PDOS not known (e.g. a simple “Top Hat” shape often gives good VBM estimates in ionic materials with Egap > 5eV) 17th International Conference on Dynamical Processes in Excited States of Solids, Argonne National Laboratory, June 2010 Systematic Trends of 4f Binding Energies The measured binding energies of the rare-earth ion 4f electrons display a characteristic trend across the 4fN series -4 YAG -3 VBM BE Relative to VBM (eV) Experimental Spectra Fit of Theory to Data Final States 7%Gd:YAG TbAG DyAG HoAG -2 -1 1 2 3 4 30%Er:YAG 5 LuAG 20 PES Data Optical Data This “zig-zag” trend is related to variation in effective nuclear charge, inter-electronic repulsion, 4f spin-pairing energy, and spinorbit coupling 50%Yb:YAG 22 YAG Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu TmAG 24 Band Gap Valence Band 0 18 16 14 12 10 Binding Energy (eV) 8 6 4 First quantitatively described by Jørgensen’s Refined Spin-Pairing Energy Theory in 1962 17th International Conference on Dynamical Processes in Excited States of Solids, Argonne National Laboratory, June 2010 Electron Binding Energies in Ionic Crystals Model the 4f electron binding energy as the free-ion value shifted by (mostly) electrostatic interactions with each host lattice Free-ion 4f electron energy (~40 eV) modified by electrostatic potential, or Madelung potential (~30 eV), of crystalline environment [Pauling 1929] Covalency modifies effective ionic charges and the Madelung potential (~1 eV to ~15 eV) [Fadley, Hagstrom, Klein, & Shirley 1968] Lattice polarizability screens charges and stabilizes ionized final state (~5 eV) [Mott & Littleton 1938] Change in inter-atomic Born repulsive energy (~0.5 eV to 1 eV) [Citrin & Thomas 1972] Change in van der Waals interaction and vibrational zero-point energies (~0.5 eV) [Poole, Szajman, Leckey, Jenkin, & Liesegang 1975] Distortion of wavefunctions, central-field covalency, nephelauxetic effect, … (few eV?) [Jørgensen 1962] For doped materials, the impurity-induced distortion of the lattice site affects all of these energy terms (< 5 eV) [Pedrini, McClure, & Anderson 1979] 17th International Conference on Dynamical Processes in Excited States of Solids, Argonne National Laboratory, June 2010 An Empirical Model for 4f Binding Energies A simple two-parameter semi-empirical form of the electrostatic model accurately describes relative 4fN energies of all rare-earth ions in a material 0 Conduction Band Electron Binding Energy (eV) 2 The chemical shift consists of a large constant shift (EL) and a smaller shift that depends on ionic radius (aR) [Pedrini, McClure, & Anderson 1979] 4 6 Egap 3+ RE :LaF3 8 4f 10 N 12 14 Model the 4f binding energies as freeion values shifted by interactions with the lattice—“Chemical Shift” Valence Band 16 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu If we treat the model parameters EL and aR as empirical values that must be measured, they then predict 4f binding energies for all fourteen rare earths in a host [Thiel et. al 2001] We found that this simple approach is very successful across a broad range of materials [Thiel et. al 2002, 2003] This model is successful for rare earths due to their chemical similarity, small variation in ionic radii, and the shielded, non-bonding character of 4f electrons 17th International Conference on Dynamical Processes in Excited States of Solids, Argonne National Laboratory, June 2010 Using Host PES to Predict 4f Energies A consequence of the electrostatic model is that all core electrons of any chemically similar ions should experience the same chemical shift When we substitute rare-earth impurity ions for similar host cations such as Y3+ or La3+, the measured chemical shift of the host cation’s core electrons gives us an estimate of EL We find that using a fixed value of aR ~ 10 eV/Å in the empirical model gives a sufficient degree of accuracy for many optical materials Analysis of photoemission measurements on undoped host crystals indicate that this approach predicts the 4f electron binding energies for rare-earth dopants to within the experimental accuracy of ~0.5 eV Material Peak EBE (exp.) EL (est.) EL (exp.) LiYF4 LiYF4 YF3 YF3 LaCl3 LaBr3 YAG YAG Y3+ 4s Y3+ 3d5/2 Y3+ 4s Y3+ 3d5/2 La3+ 4d5/2 La3+ 4d5/2 Y3+ 4p3/2 Y3+ 3d5/2 52.4 eV 164.7 eV 51.6 eV 164.3 eV 108.8 eV 108.8 eV 30.4 eV 162.5 eV 30.1 eV 29.8 eV 30.9 eV 30.2 eV 30.8 eV 30.8 eV 31.4 eV 32.0 eV 29.9 eV 29.9 eV 30.2 eV 30.2 eV 31.2 eV 31.3 eV 31.5 eV 31.5 eV 0.2 eV -0.1 eV 0.7 eV 0.0 eV -0.4 eV -0.5 eV -0.1 eV 0.5 eV This simple estimation method works well! 17th International Conference on Dynamical Processes in Excited States of Solids, Argonne National Laboratory, June 2010 Non-ionic Materials The model may be tested using 4f electron binding energies in the elemental rare-earth metals, the opposite extreme from ionic insulators 4 Elemental Rare-earth Metals Binding Energy (eV) 5 6 The 4f binding energies are known very accurately in the elemental metals—no charging effects and negligible vibrational broadening [Lang et. al 1981] The PES structure establishes that metals have same 4fN configurations as trivalent ions, except Eu and Yb 7 8 9 10 EF=3.3 eV EL=34.2 eV aR=17.9 eV/Å 11 Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu The simple two parameter model is successful for materials ranging from metals to ionic insulators The model is remarkably successful in describing the relative 4f energies of the rare-earth metals Considerations of effective charge and electronic screening in covalent or metallic materials leads to a similar form for the binding energy variations [Thiel 2002] 17th International Conference on Dynamical Processes in Excited States of Solids, Argonne National Laboratory, June 2010 The 4fN-15d Binding Energies Combining 4fN to 4fN-15d transition energies with 4fN binding energies gives the binding energies of the 4fN-15d states 0 Conduction Band Electron Binding Energy (eV) 2 4 N-1 4f 5d N-1 Measured 4f 5d Energies 6 LaF3 8 10 N-1 Model For Lowest 4f 5d Energies N Measured 4f Energies N Model for 4f Ground State Energies N 4f 12 Valence Band 14 16 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu The lowest 4fN to 4fN-15d transitions in a material may be accurately described using a oneparameter empirical model [Dorenbos 2001] The model for the 4fN-15d transition energies may be combined with the 4fN binding energy model to give a simple three-parameter empirical model that describes both the 4fN and 4fN-15d binding energies [Thiel et. al 2002] Similar behavior is expected for other mixed configurations such as 4fN-16s and 4fN-16p These results show that the 4fN-15d binding energies are similar for all rare-earth ions in a host material with maximum variations of 0.5 eV 17th International Conference on Dynamical Processes in Excited States of Solids, Argonne National Laboratory, June 2010 Inverse Photoemission and the 4fN+1 States Binding Energy Relative to Fermi Energy (eV) Inverse Photoemission Spectroscopy (IPES) is the time reverse of PES and measures binding energies of unoccupied electron acceptor states The same empirical model used for 4fN states may be applied to 4fN+1 -6 Elemental Rare Earth Metals -4 N+1 4f EL=21.4 eV N+1 4f a=8.3 eV/Å -2 0 Valence Band "Divalent" 4f Configuration N 2 4 Polarization of lattice decreases the 4fN binding energy and increases the 4fN+1 binding energy N 4f N 6 Energy difference between 3+ and 2+ states is not as large for ions in solids (~5-9eV) as for free ions (~15-20eV) 4f EL=34.2 eV a=17.7 eV/Å "Trivalent" 4f Configuration N 8 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu IPES and PES values from Lang, Baer, & Cox 1981 In metals EL = -12.8 eV, and this estimate predicts EL = -13.3 eV In ionic materials, this rough estimate has errors up to 10-20% due to neglect of other terms in model 17th International Conference on Dynamical Processes in Excited States of Solids, Argonne National Laboratory, June 2010 The 4fN+1 States and Charge Transfer Understanding the location of 4fN+1 acceptor states relative to the host valence band is critical to understand charge transfer transitions [Happek et. al 2001] N+1 Electron Binding Energy (eV) 0 4f Conduction Band 2 4 Optical Data 6 8 10 LaF3 Charge Transfer Transitions IPES Data N+1 4f Model 12 14 Valence Band We may compare IPES results to measured charge transfer energies to determine the regions of the valence band density of states (DOS) that have largest transition probability to the RE 4f orbitals In ionic materials, the anion ligands have the greatest DOS near the VBM This type of model was developed and applied over a wide range of materials to describe and predict relative RE charge transfer energies [Dorenbos 2003] 16 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu IPES data from Park & Oh 1993 CT Absorption Data from Heaps, Elias, & Yen 1976, Yang & DeLuca 1978, Krupa, Gerard, & Martin 1993 It has also been found that the aR parameter in the 4fN model has the same value for the 4fN+1 states if same set of radii are used for both [van der Kolk & Dorenbos 2006] 17th International Conference on Dynamical Processes in Excited States of Solids, Argonne National Laboratory, June 2010 Effect of Lattice Relaxation To understand electron transfer processes, it is essential to understand how the lattice relaxes for the different states involved [de Boer & van Geel 1935] U (Q) = E0 - AQ + 12 M w2Q2 Q0 = A M w2 ER = 1 2 M w2D Q02 Ionization Threshold Excited State Energy ESA PES PC Ground State Configurational Coordinate Q Different ionization thresholds are measured by photoemission (PES), excited-state absorption (ESA), and photoconductivity (PC) techniques Relaxation of the total adiabatic energy of the ion & lattice may be approximately described for linear electron-lattice coupling using configurational coordinate diagrams After a change in electronic state, the equilibrium position of ligands shifts on the timescale of the lattice vibrational frequencies In the few cases studied in detail, the 4fN-1+e- ionized state relaxation energy is ~2-4 times larger than for 4fN-15d with the same sign of Q For ionized states, the optical and DC dielectric constants can be used to estimate the relaxation energy [Mott & Littleton 1938] 17th International Conference on Dynamical Processes in Excited States of Solids, Argonne National Laboratory, June 2010 Example Analysis for Pr3+:YAG Experimental Energy Diagram for Pr3+:Y3Al5O12 3+ 3 + - Pr ( H4) + h + e 8 7 4+ 2 Energy (eV) - Pr ( F5/2) + e 6 5 3+ 1 Pr (4f 5d) 4 3 2 3+ 3 1 Pr ( H4) Pr3+:YAG 0 0 Configurational Coordinate This picture successfully explains all the observed processes in Pr3+:YAG Configurational coordinate energy curves are shown for 4fN (blue), 4fN-15d (red), and host band gap (crosshatched region) The ionized Pr4+ state is indicated by the shaded region The vertical “frozen lattice” energy of ionized Pr4+ was determined from our PES results The measured relaxation energy of ionized states in RE3+:YAG is ~1.4 eV [Mayolet et. al 1995], which compares well with the calculated value of ~1.6 eV The observed ESA threshold of photoionization from the lowest 4fN-15d state [Cheung & Gayen 1994] is plotted as an arrow The dashed line is the energy where photoconductivity has been observed [Wittmann & Macfarlane 1996] 17th International Conference on Dynamical Processes in Excited States of Solids, Argonne National Laboratory, June 2010 Material Trends for 4f Electron Energies Material trends may be identified from analysis of 4f electron binding energies over a wide range of materials 6 3+ Ce 4f electrons 8 Valence Band Maximum 9 10 0 11 CeF3 CeCl3 CeBr3 Variation in the relative energies of 4f electron and host band states between materials is mostly due to shifts in the host bands, with weaker shifts in the 4f electron energies observed 3 4f N-1 5d 4 5 6 7 8 9 VB 10 11 4f N 12 13 Ce 17th YIG CB 2 12 YGG YAG 1 Binding Energy (eV) Binding Energy (eV) 7 Rare-earth impurity concentration has no observable effect on the 4f binding energies when substituting for Y3+ or other RE Crystal structure weakly affects 4f energies Changing cations with the same valence has a weak effect on 4f binding energies Changing anions has a significant effect Binding energies decrease as covalency increases Gd Lu Ce Gd Lu Ce Gd International Conference on Dynamical Processes in Excited States of Solids, Argonne National Laboratory, June 2010 Lu A Final-state Model for Material Trends The electrostatic model predicts that material trends are dominated by initial-state energy effects (e.g. Madelung potential), but this generally does not agree well with observed trends in optical materials We find that electrostatic model calculations overestimate energy differences between materials and even predict the wrong sign for some material trends (RE-halides) Measured Chemical Shift (eV) 35 Metals EL = 24.8 eV – 1.31·Epol 34 33 This is partly explained by changes in bonding covalency and ligand distances tending to compensate initial-state energy variations Oxides 32 Chlorides From these considerations, we compare 4f binding energies to a simple empirical model that only includes lattice polarization effects 31 Fluorides 30 –3.5 –4.0 –4.5 –5.0 –5.5 –6.0 –6.5 –7.0 Calculated Polarization Energy (eV) This simple model is surprisingly successful suggests that 4f electron energies may be accurately predicted using only the host crystal’s index of refraction –7.5 Using the simple Mott-Littleton approximation for Epol that only requires the index of refraction, we find a good linear correlation with the observed material trends 17th International Conference on Dynamical Processes in Excited States of Solids, Argonne National Laboratory, June 2010 Conclusions Photoemission and Inverse Photoemission spectroscopy are powerful tools for determining 4fN and 4fN+1 electron energies relative to host band structure The 4fN-15d energies relative to host bands can be found from the 4fN binding energies and the 4fN to 4fN-15d transition energies Simple models may be used to describe and predict all 4fN, 4fN-15d, and 4fN+1 energies in a host from measurements on one or two ions Understanding lattice relaxation for ionized and reduced states is critical for predicting electron transfer processes and the stability of these states ESA energies, photoionization thresholds, thermal activation energies, etc. may be obtained by measurement of 4fN and 4fN-15d energies relative to host states A simple empirical model predicted the material dependent trends in 4fN binding energies of rare-earth-ions over a wide range of materials, but further testing is required to confirm the success of this model for additional material systems Acknowledgements This material is based on work supported by the Air Force Office of Scientific Research under Grants F49620-97-1-0411, F49620-98-1-0171, and F49620-00-1-0314, Scientific Materials Corporation, and the National Science Foundation under Grants 0903937 and DGE-4189-9553556. 17th International Conference on Dynamical Processes in Excited States of Solids, Argonne National Laboratory, June 2010