485-organizational-meeting-Fall
advertisement

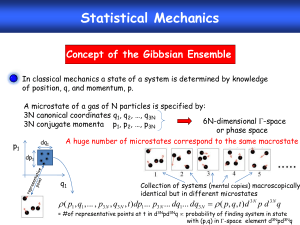
PHYS 485 General Information Physics 485 provides an introduction to quantum physics including suitable for majors in Physics, Electrical Engineering, Materials Science, Chemistry, and related Sciences. When: Lectures on Mondays and Wednesdays 2:00 - 3:20 pm. Midterms: Monday, Oct-14th and Wed, Nov-20th Final: TBA Where: Lectures and exams take place in: 136 Loomis Laboratory of Physics PHYS 485 Contact Information Instructor: Office: Phone: Email: Office hours: Professor Matthias Grosse Perdekamp 469 Loomis Laboratory (217) 333-6544 mgp@illinois.edu Tuesday 5:00-6:00pm Grader: Office: Phone: Email: Tsung-Han Yeh Loomis-MRL Interpass 390F no office phone tyeh6@illinois.edu Office hours: Tuesday 4:00-5:00pm For course related e-mail: if you would like a prompt reply make sure to place “PHYS 485 “ into your subject line Course web-page: PHYS 485 Grading Policy Course grades will be determined by the following percentages: Problem sets Midterm I Midterm II Final exam 45% 15% 15% 25% Final grade boundaries will be chosen such that NA++NA+NA- ~ 40% of NAll and similar for B letter grades . PHYS 485 Homework 10 problem, one per week. Problem sets will account for 45% of the final grade. Problem sets will be distributed by e-mail Wednesdays by the end of the day and are due one week later, Wednesday in class. Late submission: 485 homework box, 2nd floor. Late deductions: 20% Wednesday 2.05pm to Thursday 6pm 40% after Thursday 6pm to Friday 6pm 100% after Friday 6pm Solutions will be posted on the course web-page on Monday morning and homework will be returned during the Monday lectures. First homework: Wednesday Sep. 4th due Wednesday Sep. 11th. Problem sets aim to enhance your learning of the material. I encourage you to consult with other students in the class on the problem sets, but remember that you will be on your own in the exams. TA and lecturer office hours are scheduled Tuesday afternoon. PHYS 485 Exams Midterms: There will be two midterm examination, given in class. Each midterm will account for 15% of your final grade. Midterm I Midterm II (Monday, October-14, in class) (Wednesday, November-20, in class) Final Exam: There will be a three-hour final exam, which will account for 25% of your final grade. The final exam will cover all course material. All exams are closed book. However, it is permitted to use a one page summary of your own notes during the midterms and three pages for the final. Calculators will be necessary. About half of the exam problems will be taken from study lists of problems for each exam and the homework. Recommended Reading Textbook: Quantum Physics of Atoms, Molecules, Solids, Nuclei, and Particles , 2nd Edition, Robert Eisberg and Robert Resnick (1985). Other books you might want to consult: Quantum Physics, 3rd Edition, Stephen Gasiorowicz (2003). The Feynman Lectures on Physics, Vol.III, R. Feynman, R. Leighton, M. Sands (1964). As Reference for selected topics: Quantum Mechanics, C. Choen-Tannoudji, B. Diu, F. Laloe (1992). PHYS 485 Makeup Time & Day Poll: What is the best time to schedule a Makeup Class if necessary? [I will try to avoid this but it might become necessary ~ 2 times, to acommodate travel to my experiment at BNL] Tuesday, Thursday, Friday 2-3.20pm 3-4.20pm 4-5.20pm 5-6.20pm 6-7.20pm 7-8.20pm 8-9.20pm Please raise your hand for times that will not work for you! Quantum Mechanics Scope: Quantitative description of phenomena observed in atoms, molecules, nuclei, elementary particles and condensed matter (in the non-relativistic limit). The goals of this course are to (a) review the basic concepts of quantum mechanics (b) study it’s applications for a broad range of different areas: atoms, molecules, condensed matter and nuclei. Why Quantum Mechanics ? In the late 19th and early 20th century physics experiments increasingly gain access to microscopic observables: However, attempts to describe atomic particles as point masses governed by the laws of classical mechanics and field theory (E&M) fail for an increasing set of experimental observations. Examples: Thermal radiation : “ultraviolet catastrophe” for black body radiators ( Wednesday!) Atomic spectra : discrete optical emission lines! Intrinsic orbital angular momentum: spin phenomena, eg. Stern Gerlach experiment can not be explained in the framework of classic physics Superconductivity : again, no classical explanation A Current Example on How Well Classical Physics Works! Classical EM allows perfect description of currents and voltages in case of an events that leads to the loss of superconductivity in the g-2 magnet: Fit of classical transformer eqns. to highly precise data ! World largest superconducting solenoid upon arrival at Fermi-Lab in July 2013 Why Quantum Mechanics ? In the late 19th and early 20th century physics experiments increasingly gain access to microscopic observables: However, attempts to describe atomic particles as point masses governed by the laws of classical mechanics and field theory (E&M) fail for an increasing set of experimental observations. Examples: Thermal radiation : “ultraviolet catastrophe” for black body radiators ( Wednesday!) Atomic spectra : discrete optical emission lines! Intrinsic orbital angular momentum: spin phenomena, eg. Stern Gerlach experiment can not be explained in the framework of classic physics Superconductivity : again, no classical explanation The Stern Gerlach Experiment T hemagneticmoment of theAg atom μS is proportion al to thespin of theoutermost (5s) electronspin S . T heforceon thesilver atomsis B Fz z z z In classic physicstheAg magmenticmoments in theovencan be orientedin any direction resultingin a continousdistribution of z . The Stern Gerlach Experiment Interesting reading: Physics Today article online Phys. Today 56(12), 53 (2003); doi: 10.1063/1.1650229 View online: http://dx.doi.org/10.1063/1.1650229 Classic Physics vs Quantum Mechanics I Classical mechanics Classic Physics vs Quantum Mechanics II Electrodynamics Features of classical particles and waves: deterministic equations well defined quantities measurements can (in principle) be precise and non-invasive Classic Physics vs Quantum Mechanics III Quantum mechanics Classic Physics vs Quantum Mechanics IV Features of systems governed by quantum physics wave-particle duality interference uncertainty principle (fundamental limit on measurements) quantization of energy levels (atomic structure) entanglement (Schroedinger’s cat paradox, Einstein, Podolsky Rosen, EPR, paradox) quantum statistics (Pauli exclusion principle, Bose condensation) condensed matter (superconductivity) Interpretation/philosophical issues interaction of measurements on system evolution causality, determinism Historical benchmarks in the development of Quantum Mechanics Historical benchmarks in the development of Quantum Mechanics