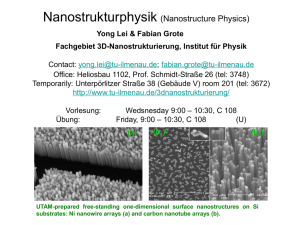
Wide Bandgap Semiconductor Nanowires for Sensing
advertisement
Wide Bandgap Semiconductor Nanowires for Sensing • S.J. Pearton1, B.S. Kang1, B.P.Gila1, D.P. Norton1, L.C.Tien1, H.T.Wang2, F. Ren2, ChihYang Chang3,G.C. Chi3,Wei-Ming Wang3 and LiChyong Chen4 • 1Department of Materials Science and Engineering, University of Florida, Gainesville, FL 32611-6400, U.S.A • 2Department of Chemical Engineering, University of Florida, Gainesville, FL 32611, U.S.A. • 3Department of Physics, National Central University, Jhong-Li 320, Taiwan • 4Center for Condensed Matter Sciences, National Taiwan University, Taipei 106, Taiwan GaN Applications Blue/violet/white/UV LED Blue/green/UV lasers High power microwave transistors Robust sensors GaN NWs grown by catalytic chemical vapor deposition FESEM image & CL spectrum of a single GaN NW with two electrodes 500μm 5μm Ti/Au Pad SiNx/Si Ti/Au Pad C L Intensity (a. u.) 7000 sin g le G a N N W th e p e a k p o sitio n : 3 .4 7 e V 6000 5000 4000 2 .8 3 .0 3 .2 3 .4 3 .6 E n e rg y (e V ) 3 .8 4 .0 4 .2 Gate voltage-dependent I-Vsd curves of a single GaN NW The carrier mobility is estimated at 30 cm2/V·s. The carrier concentration is estimated to be 2×1017 cm-3 InN NWs grown by catalytic thermal-CVD HRTEM image 30 35 40 (440) 45 50 2 (d e g re e ) XRD spectrum (103) (110) (102) (413) (332) In (110) (411) (400) (002) (222) (100) 25 5 nm In N In 2 O 3 In te n s ity (a .u .) 0 .3 08 n m (101) (a ) 55 60 Temperature-dependent I-V curve of a InN NW 0 .0 0 0 6 0 .0 0 0 4 C u rre n t (A ) 0 .0 0 0 2 0 .0 0 0 0 -0 .0 0 0 2 In N C 6 3 -9 4 -9 400K 350K 300K 250K 200K 150K 100K 50K 4K -0 .0 0 0 4 -0 .0 0 0 6 -1 .0 -0 .5 0 .0 V o lta g e (V ) 2300 2250 R e sista n ce ( ) 2200 D a ta : R vs T _ B M o d e l: T E M c o e f. o f re s is tivity C h i^2 R ^2 = 2 0 5 .7 7 1 4 3 = 0 .9 8 1 7 R0 2 1 4 5 .1 1 4 2 9 0 .0 0 0 4 7 300 ±0 2150 T0 ± 6 .1 0 2 0 3 ± 0 .0 0 0 0 3 2100 2050 2000 In N C 6 3 -9 4 -9 R = R 0 [1 + (T -T 0 )] 1950 50 100 150 200 250 300 T e m p e ra tu re (K ) 350 400 450 0 .5 1 .0 Resistivity comparison between thin film and nanowire (n-type GaN and InN) thin film resistivity (Ω cm) nanowire contact resistivity resistivity (Ω contact resistance (Ω cm2) cm) (Ω) n-GaN 4.4×10-2 a 3~7×10-6 a,b 56 ~ 1.24×10-4 c,d,e InN 2.1~ 3.1×10-3 1.8×10-7 f 4×10-4 i X 2i f,g,h a Solid State Electron 41, p165-168 (1997) b Appl. Phys. Lett. 70, p57-59 (1997) c Appl. Phys. Lett. 85, p1636-1638 (2004) d Nano Lett. 2, p101-104 (2002) e Nano Lett. 3, p1063-1066 (2003) f Appl. Phys. Lett. 64, p1508-1510 (1994) Solid-state Electronics, 39, p1289-1294 (1996) h J. Vac. Sci. Technol. B, 14, p3520-3522 (1996) i This work g Single Crystal Nanowire 0002 1010 • TEM image of an individual ZnO Nanowire. • An estimated diameter of the wire is 20 nm. • A small particle embedded at the tip of the wire is Ag or Ag-Zn alloy. • HR-TEM image and selected area diffraction (SAD) of the nanowire indicates that it is a single crystal ZnO. Heterostructured nanowires Radial heterostructure Type I Sheath (Zn,Mg)O (Hexa.) Axial heterostructure Type II Core (Zn,Mg)O (Hexa.) Growth condition -. Zn : 3 × 10-6 mbar -. Mg : 2 × 10-7 mbar -. O3/O2 : 5 × 10-4 mbar, -. Tg= 400C (Mg,Zn)O (cubic) Zn1-xMgxO (x <0.02) (Hexa.) Growth condition -. Zn : 3 × 10-6 mbar -. Mg : 4 × 10-7 mbar -. O3/O2 : 5 × 10-4 mbar, -. Tg= 400C ZnO (Zn1-XMgX)O ZnO (Zn1-XMgX)O Type I - Radial heterostructured nanowire Sheath (Zn,Mg)O (Hexa.) Core (Zn,Mg)O (Hexa.) -. Nanowire is crystalline with the wurtzite crystal structure maintained throughout the cross- section. -. The higher contrast for the center core region clearly indicates a higher cation atomic mass. -. Core : zinc-rich Zn1-xMgxO -. Sheath : Mg-rich Zn1-yMgyO Type II - Radial heterostructured (Zn,Mg)O/(Mg,Zn)O nanowire a (Mg,Zn)O (cubic) bb Zn1-xMgxO (x <0.02) (Hexa.) 10 nm Compositional line scan across the nanowire (STEM) (11ī) -. Sheath (Shell): Mg1-xZnxO Cubic Rock salt structure Zn 140 Mg Zn Mg 120 N o rm a liz e d C o u n ts -. Core : Zn1-xMgxO Hexagonal Wurtzite structure 100 80 60 40 20 0 0 10 20 30 40 50 P o s itio n a c ro s s N a n o w ire (n m ) 60 (Mg,Zn)O nanowire (cubic rock salt structure) 020 200 2.04 Å B=[001] M gO Intensity(arb.) In te n s ity (a rb .) 80 Growth condition Mg O Zn Mg -. Zn : 3 × 10-6 mbar 60 -. O3/O2 : 5 × 10-4 mbar, 40 O -. Mg : 8 × 10-7 mbar 20 -. Tg = 400C Zn 0 0 50 100 150 P o sitio n a cro ss th e n a n o w ire (n m ) Position across nanowire(nm) 200 Nanowires vs Zn, Mg pressures ZnO Radial heterostructured (Zn,Mg)O I hexagonal wurtzite st. core / sheath (Zn1-xMgx)O/(Zn1-xMgx)O hexa. / hexa. wurtzite / wurtzite (Mg,Zn)O II core / sheath (Zn1-xMgx)O / (Mg,Zn)O cubic rock salt st. hexa. / cubic wurtzite / rock salt st. Zn = 3 × 10-6 Zn = 3 × 10-6 Zn = 3 × 10-6 Zn = 3 × 10-6 O3/O2 = 5 × 10-4 O3/O2 = 5 × 10-4 O3/O2 = 5 × 10-4 O3/O2 = 5 × 10-4 Mg = none Mg = 2 × 10-7 Mg = 4 × 10-7 Mg = 8 × 10-7 Tg= 400C [unit: mbar] Fabrication of ZnO nanowire device ZnO Nanowire Electrode (Al/Pt/Au) Al/Pt/Au Motivation -. Fundamental understanding of transport -. Nanoelectronics Insulator -. Nano sensors (UV, chemical, bio.) Structure of Nanodevice -. Electrode : Al/Pt/Au by sputtering -. Diameter of ZnO nanowire : 130 nm -. Channel Length : 3.7 m Prototype device fabrication sequence Find Nanowires Relative To Alignment Marks Deposit SiO2 Spin PMMA Resist Design and Deposit Alignment Marks Ethanol and Nanowire Suspension E-beam Write Aligned Pattern And Develop Deposit Metal And Lift Off Evaporation & Nanowires Deposition UV Response of single ZnO nanowire U V = 3 6 6 n m re sp o n se a t 0 .2 5 V UV 366nm at VD 0.25V UV 366nm D a rk UV366nm 800 C u rre n t (n A ) 20 Dark 0 -2 0 C u rre n t (n A ) 40 on 600 400 200 -4 0 off 0 -0 .3 -0 .2 -0 .1 0 .0 0 .1 V o lta g e (V ) 0 .2 0 .3 0 100 200 T im e (se c) 300 400 Pt/ZnO nanowire Schottky Diode Pt/Au (schottky contact) 0 .9 D a rk UV366nm Al/Pt/Au Al/Pt/Au C u rre n t (n A ) 0 .6 0 .3 0 .0 -0 .3 -0 .6 -0 .9 -0 .4 -0 .2 0 .0 0 .2 0 .4 B ia s (V ) 1x10 0 .0 0 1 Forward Bias Reverse Bias 1x10 C u rre n t (n A ) C u rre n t (n A ) -0 .0 5 -0 .1 0 1x10 1x10 -0 .1 5 1x10 -0 .2 0 -1 0 1x10 -8 -6 -4 B ia s (V ) -2 0 0 -1 -2 I=Io(eqV/nkT-1) Ideality factor = 1.1 -3 -4 0 .0 0 0 .0 5 0 .1 0 B ia s (V ) 0 .1 5 0 .2 0 Depletion-mode ZnO nanowire field-effect transistor Gate oxide ((Ce,Tb)MgAl11O19) Source (Al/Pt/Au) Gate(Al/Pt/Au) 8x10 Nanowire -8 V G=0 V Drain (Al/Pt/Au) V G = -0 .5 V 6x10 V G = -1 V -8 V G = -1 .5 V V G = -2 V I D S (A ) V G = -2 .5 V Insulator (SiO2) 4x10 2x10 Si -8 -8 0 0 2 4 6 8 10 V D S (V ) Gate 6x10 -8 ID S Oxide 4x10 -8 0.2 I D S (A ) Drain 2x10 Nanowire 0.1 -8 0.0 0 -3 -2 -1 V G (V ) 0 g m (m S /m m ) Source 0.3 gm pH Sensing with Single ZnO Nanowire electrode (Al/Pt/Au) Nanowire 1 .6 x1 0 -7 non UV U V (3 6 5 n m ) 1 .2 x1 0 -7 I D S (A ) Microchannel Insulator (SiO2) 8 .0 x1 0 -8 2 4 .0 x1 0 3 4 -8 5 6 7 8 9 10 11 12 0 .0 Si 0 100 200 300 400 500 600 T im e (s e c ) C o n d u c ta n c e (n S ) 300 non UV U V (3 6 5 n m ) 250 200 150 100 50 0 2 3 4 5 6 7 pH 8 9 10 11 12 Hydrogen Detection • • • • Hydrogen has been used as fuels in many NASA’s space exploration missions. President Bush’s Hydrogen Fuel Initiative in 2003. Why hydrogen sensing? – Safety! – Production, Storage, Transport Hydrogen concentration in air reaches a dangerous level at 4%. ppm-level detection is needed. Simple Fabrication Process • Direct deposition of metal contacts on the silicon substrate with nanorods. • No need to go through sonication and E-beam lithography to fabricate the sensors. • The sensor has better sensitivity (more nanorods combined). Al/Pt/Au Al/Pt/Au Hydrogen-Selective Sensing at Room Temperature with ZnO Nanorods Hydrogen-selective gas sensing at 25C with Pd/ZnO nanorods 960 Resistance(ohm) ZnO nanorod without Pd 950 N2 O 2 10ppm H2 Air 100ppm H2 Air 250ppm H2 Air 500ppm H2 Air 670 660 650 ZnO nanorod with Pd 640 0 30 60 90 Time(min) 120 150 Wireless Hydrogen Sensor System Prototype – powered by Remote Sensor Central Station battery Low-noise Op Amp Microcontroller RX TX 916 MHz Microcontroller 16x1 LCD Self-Powered Wireless Sensor • Use energy from ambient – Solar, vibration, ambient RF radiation • Use energy supplied locally – Hydrogen flow, micro fuel cell, acoustic, thermal gradient • Use energy supplied remotely – Wireless power supply (wireless power transmission) Conclusions High quality,single-crystal growth of wide bandgap semiconductor nanowires Bimodal growth of cored ZnO/(Zn,Mg)O heterostructured nanowires. Type I -. Core : Zn1-xMgxO (x < 0.02) , Hexagonal wurtzite structure -. Sheath : Zn1-xMgxO (x >> 0.02), Hexagonal wurtzite structure Type II -. Core : Zn1-xMgxO (x < 0.02), Hexagonal wurtzite structure -. Sheath : (Mg,Zn)O, Cubic rock salt structure (Mg,Zn)O nanowires having cubic rock salt structure Functional Nano-devices Pt/ZnO nanowire Schottky Diode Depletion-mode GaN and ZnO nanowire field-effect transistor UV, pH, & gas sensors from GaN,InN and ZnO nanowires