Jan 21 Atmospheric structure & Composition (Chapter 3)

The
Atmosphere
It appears that approximately an inch (2.5 cm) of ice is coating this hapless tree. The icy fangs are an indication that melt is underway. Typically, a quarter inch (0.65 cm) of ice is all that ’s needed for tree branches to begin to snap.
Frequency, Wavelengths & Energy of
Photons
Energy emitted from the sun
(i.e electromagnetic radiation exhibits both a wave-like
(electromagnetic wave) and a particle-like (photon) nature.
Duality of light and matter
In 1690 Christiaan Huygens theorized that light was composed of waves, while in 17-4 Isaac Newton explained that light was made of particles. Experiments supported each of their theories. However neither a completelyparticle theory nor a completely-waver theory could explain all of the phenomena associated with light.
So how can something be both a particle and a wave at the same time?
The wave of light is simply the probability of where the particle will be.
Let ’ s hear a little quantum physics explained.
Energy in the form of photons is absorbed or emitted as electrons change energy levels within the structure of an atom.
Photon = A particle-like unit of electromagnetic energy (light) emitted or absorbed by an atom when an electrically charged electron changes state.
Recall that
Photons are energy packets having a well-defined wavelength and frequency.
a) An electron in its ground state about to absorb a photon b) The electron leaps to a higher level as the photon is absorbed.
As an electron emits or “ gives off ” electromagnetic energy (in the form of a photon), it jumps from a Higher to a Lower energy state
(level).
a) An electron in its excited or quantized state.
b) The electron leaps to a lower energy level and a photon is emitted.
Summary of quantum mechanics & the link to absorption of electromagnetic energy at the subatomic scale.
If a photon of electromagnetic energy strikes an atom,
And if the Frequency of the electromagnetic radiation is such that it is equal to the difference in energy of the ground level
& the first excited level,
The electron Absorbs the photon energy and….
The electron is “ moved ” (quantum leap) to “ Level 2 ”
Quantum behavior of Molecules
Quantum theory also involved the behavior of molecules: the molecular-scale motion (I.e. rotation, bending, & vibration) of molecules.
Molecular motions in the gases Water Vapour and
Carbon Dioxide ( H
2
O and CO
2
) explain why some gases contribute to the greenhouse effect and others do not.
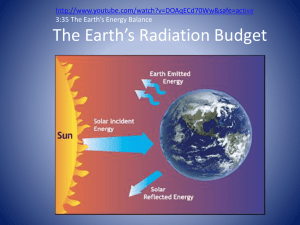
Both Sun & Earth are radiating energy…
…At different electromagnetic wavelengths
….and at different frequencies
Wavelengths
Quantifying Frequency & Wavelengths
First we ’ ll talk about the WAVE-like behavior of electromagnetic energy:
Wavelength = the distance between adjacent crests (or troughs) (symbol = lambda
)
Frequency = how fast the crests move up and down (symbol = nu
)
Speed = how fast the crests move forward
(symbol = c ) the speed of light
The pattern of wavelengths absorbed by a particular atom or combination of atoms, (e.g. a gas molecule of CO
2 or H
2
O)
Is called its Absorption Spectrum or its absorption curve
Solar
Radiation greatest intensity in
SHORT wavelengths
(high energy & frequency)
Earth
(terrestrial)
Radiation entirely in
LONG wavelengths
(low energy & frequency)
Quantum behavior of Molecules
Quantum theory also involved the behavior of molecules: the molecular-scale motion (I.e. rotation, bending, & vibration) of molecules.
Molecular motions in the gases Water Vapour and
Carbon Dioxide ( H
2
O and CO
2
) explain why some gases contribute to the greenhouse effect and others do not.
Nitrogen Gas
Molecule
N
2
Water Molecule
H
2
O
Carbon Dioxide
Molecule
CO
2
Nitrogen Gas
Molecule
N
2
Not a Greenhouse Gas
Water Molecule
H
2
O
Greenhouse Gases
Carbon Dioxide
Molecule
CO
2
When the H
2
O molecule emits a photon its rotation rate decreases.
When it absorbs a photon, the rotation rate increases.
Molecules can also absorb and emit IR radiation by changing the amplitude with which they vibrate.
If the frequency at which a molecule vibrates matches the frequency of an electromagnetic wave , the molecule can absorb a photon and begin to vibrate more vigorously.
As a triatomic molecule, one way that CO
2 vibrates is in a
“ bending mode ” that has a frequency that allows CO
2 to absorb IR radiation at a wavelength of about 15 micrometers.
Another triatomic molecule: N
2
O does the same thing.
Dance your PhD
N
2
O acts as a greenhouse gas through the absorption of radiation in 3 vibrational modes.
With one hand as a nitrogen atom, torso as central nitrogen, and the other hand as an oxygen atom, the dancers exhibit the three specific movements of N
2
O ’ s vibrational modes as it moves from soil to atmosphere. http://www.youtube.com/w atch?v=L5j6BS3XoLc
The N
2
O starts in the soil where it is produced by microbial activity and “ moves on up ” into the atmosphere.
Stepping onto the chairs represents the progression of N
2
O to higher levels in the atmosphere (the stratosphere) where it is subject to intense Ultraviolet UV radiation from the sun.
This high energy from the bombarding UV radiation is shown in the dancers ’ high energy, more spastic dancing.
The high intenesity UV radiation leads to the destruction of N
2
O --Jumping from the chair.
We will learn later that interaction of N
2
O in the stratosphere with UV wavelengths is related to Ozone Depletion
…but N
2
O also vibrates & bends when absorbing Infrared
(IR) wavelengths
…it is the ability to absorb and emit IR radiation that makes
N
2
O a GREENHOUSE GAS
What defines a GREENHOUSE GAS ?
Abbreviation we ’ ll use = GHG
GHG = a gas that can absorb and emit (re-radiate)
Infrared wavelengths of Electromagnetic Radiation
The Quantum Behavior of certain molecules with respect to Infrared radiation is the reason that
Greenhouse Gases are
Greenhouse Gases.
Longwaves
(LW)
Shortwaves
(SW)
Key bands in the spectrum for Global Change:
UV, Visible, IR, NIR
Definition of Greenhouse Gases
Greenhouse gases are gases which both absorb and emit electromagnetic radiation in the infrared (IR) part of the spectrum.
Once IR is absorbed by the greenhouse gases in the atmosphere, it can be emitted back to the
Earth ’ s surface to heat it all over again.
Or it can be emitted upward to outer space and be lost from the system altogether.
IR radiation is absorbed by GH gases in the atmosphere and emitted out to space
IR radiation is emitted fro the
Earth ’ s surface right out to space through
“ IR window ”
IR radiation is absorbed by
GH gases in the atmosphere and emitted back to Earth.
Different gases absorb & emit radiation at different wavelengths
How do we know which wavelengths are absorbed/emitted by different gases?
The pattern of electromagnetic wavelengths that are absorbed & emitted by a particular atom (or combination of atoms)
Is called its Absorption Spectrum or Absorption Curve
Match the gas with its absorption curve.
Choices: H
2
O O
2
/O
3
N
2
O CH
4
CO
2
Match the gas with its absorption curve.
Choices: H
2
O O
2
/O
3
N
2
O CH
4
CO
2
Choices: H
2
O O
2
/O
3
N
2
O CO
2
???
Key concepts to get out of all of this:
1. Solar radiation is mostly in shortwave (SW) form (visible and
UV) .
Most visible and UV wavelengths are transmitted through the atmosphere but some (esp. harmful UV) are absorbed on their way to Earth ’ s surface by O
2
O
3
.
and
2. Most of the incoming solar energy absorbed by the Earth and the atmosphere is absorbed at the
Earth ’ s Surface which then radiates IR outward to heat up the atmospehre.
Hence, the Atmosphere is heated primarily from below
3. Terrestrial radiation is mostly in longwave (LW) form (IR).
Much of the outgoing terrestrial radiation is absorbed by H
2
CO
2
O and
(and other GHG ’ s) before it escapes to space, and it is reradiated back to the Earth ’ s surface.
This is the “ Greenhouse Effect ”
4. The re-radiation of LW (IR) energy to the Earth ’ s surface by GH gases is what keeps the Earth in the “ just right ” temperature range for water to be present in all 3 phases and just right for US.
Without the “ Greenhouse Effect ” the
Earth would be too COLD for life as we know it!
Objectives:
To understand:
The vertical structure of the atmosphere & its relationship to temperature
Which gases are in the atmosphere where they are concentrated
Why gases at different levels are linked to the greenhouse effect & ozone depletion
http://earthguide.ucsd.edu/earthguide/diagrams/atmosphere/index.html
The Vertical Structure of the
Atmosphere
Key Concept:
The atmosphere ’ s vertical structure is defined by changes in the trend of temperature with height
Atmospheric Pressure & Mass vary with Height.
Atmospheric Pressure = weight of the air column above
99 % of mass lies below
~50km ( top of
Stratosphere )
50% of mass lies below ~6 km ( middle
Troposphere )
Why the zig-zags in the temperature height graph?
The changes in temperature with height are the result of:
Differential absorption of shortwave (SW) & longwave (LW) radiation
By atmospheric gases concentrated at various altitudes.
Incoming solar SW
(mostly visible & near IR
+ UV)
Outgoing terrestrial LW
(Far IR) radiated from
Earth ’ s surface
Recall that Earth ’ s atmosphere is heated from below.
On its way to the Earth ’ s surface, several things can happen to incoming
Solar radiation
Transmitted ( to Earth ’ s surface)
Absorbed (by gases, dust, clouds)
Scattered/ Reflected reflected back to space
Scattered (and indirectly transmitted to Earth ’ s surface)
Why is the sky Blue?
Review: The pattern of electromagnetic wavelengths that are absorbed & emitted by a particular atom (or combination of atoms) is called its Absorption spectrum
(curve)
How incoming
Solar radiation of different wavelengths gets
Transmitted or
Absorbed by different gases on its way to Earth ’ s surface.
UV UV UV UV
A
Near IR
(C & B) Visible
Q1 - The atmospheric layer of the troposphere is important because:
1. It is the layer closest to the sun, which is the source of the Earth ’ s energy
2. It is the layer in which temperature increases with altitude in the atmosphere and where most of the atmosphere ’ s ozone occurs
3. It is the layer in which most of our weather, heat transfer, & greenhouse gases
Q1 - The atmospheric layer of the troposphere is important because:
1. It is the layer closest to the sun, which is the source of the Earth ’ s energy
2. It is the layer in which temperature increases with altitude in the atmosphere and where most of the atmosphere ’ s ozone occurs
3. It is the layer in which most of our weather, heat transfer, & greenhouse gases
Q2 -Here are 3 graphs showing “ something ” varying with altitude in the atmosphere. Which is which?
1. A = water vapour, B = pressure, C = Temperature
2. A = temperature, B = pressure, C = ozone concentration
3. A = ozone concentration, B = temperature in the troposphere, C = temperature in the stratosphere
Q2 -Here are 3 graphs showing “ something ” varying with altitude in the atmosphere. Which is which?
1. A = water vapour, B = pressure, C = Temperature
2. A = temperature, B = pressure, C = ozone concentration
3. A = ozone concentration, B = temperature in the troposphere, C = temperature in the stratosphere
Q3 - Here is the graph of atmospheric pressure vs. altitude, with “ parcels of air ” shown to depict the density of the atmosphere ’ s gases at 3 different altitudes. If the air in Parcel X is forced to subside (sink) to the altitude of Parcel
Z, what will happen to the air in Parcel
X?
1. It will get more dense and get cooler
2. It will get more dense and warm up
3. It will get more dense, and no change in temperature will occur
Q3 - Here is the graph of atmospheric pressure vs. altitude, with “ parcels of air ” shown to depict the density of the atmosphere ’ s gases at 3 different altitudes. If the air in Parcel X is forced to subside (sink) to the altitude of Parcel
Z, what will happen to the air in Parcel
X?
1. It will get more dense and get cooler
2. It will get more dense and warm up
3. It will get more dense, and no change in temperature will occur
Atmospheric Composition
Which gases?
What concentration?
Which ones are Greenhouse Gases (GHG)?
Where do the GHG ’ s come from?
Most abundant gases in the atmosphere
Gas
Nitrogen
Oxygen
Argon
Total
Symbol
N
2
O
2
Ar
% by volume
78.08
20.95
0.93
99.96
% in ppm
780,000
209,500
9,300
Next most abundant gases in the atmosphere
Gas
Water
Vapour
Carbon
Dioxide
Symbol
H
2
O
CO
2
Greenhouse Gases!!
% by volume
0.00001
to 4.0
0.0390
% in ppm
0.1-40,000
360
390
(in 1960)
(in June!)
Other important Gases
Gas Symbol
Methane CH
4
Nitrous
Oxide
N
2
O
% by volume
0.00017
0.00003
% in ppm
1.7
0.3
Ozone O
3
0.0000004
CFC ’ s CCl
3
F
0.00026 (Freon-11)
0.000000026
0.01
CFC ’ s CCl
2
F
2
0.00047 (Freon-12)
0.000000047
Greenhouse Gases
Absorption by ALL the gases in the atmosphere put together i.e. curve for the “ Whole Atmosphere ”
Water Vapour
Arrives in the atmosphere naturally through evaporation
& transpiration
Because of the unique quantum rotation frequency, H
2
O molecules are excellent absorbers of IR wavelengths of
12
m and longer ;
Virtually 100% of IR longer than 12
m is absorbed by H
2
O vapour and CO
2
(12
m close to the radiation wavelength of 10
m, at which most of Earth ’ s terrestrial radiation is emitted.)
IR at 12
m is absorbed
Water Vapour
H
2
O has variable concentration and residence time in the atmosphere depending on location and atmospheric circulation
At higher air temperatures H
2
O molecules collide & rebound more frequently, leading to expansion of the air & the water vapour in the air.
Hence hot climates can hold more water vapour in the air
At lower air temperatures as air gets more dense, H
2
O molecules are more likely to bond so that a phase change to liquid water or even solid ice can occur.
Hence in cooler climates, more of the available H
2
O is likely to be in the liquid or solid state on the Earth ’ s surface
Water Vapour (cont):
H
2
O is NOT globally increasing in direct response to human-induced factors, but if global temperatures get warmer, H
2
O vapour in the atmosphere will increase…
Why???
…because of more evaporation in the warmer climate!
Carbon Dioxide:
Arrives in atmosphere naturally through the natural carbon cycle
Because of the unique quantum bending mode vibration behavior of CO radiation of about 15
m
2 it absorbs electromagnetic
CO
2 is an excellent absorber of radiation of about 15
m
(15
m close to the radiation wavelength of 10
m, at which most of Earth ’ s terrestrial radiation is emitted.)
IR at 15
m is absorbed
Carbon Dioxide (cont)
Has increased dramatically since the 1800s because of:
1. Fossil fuel combustion: oil, coal, gas --especially coal, and
2. Deforestation -- which has the effect of increasing the amount of carbon in the atmospheric
“ reservoir ” by reducing the photosyntesis outflow and increasing the respiration inflow.
Carbon Dioxide: Trends
Carbon Dioxide: Trends
Carbon Dioxide (cont):
Residence time in the atmosphere of carbon atoms in the carbon cycle = ~ 12.7 years;
But residence time of CO
2 about 100 years gas molecules is estimated at
Plus it takes 50 to 100 years for atmospheric CO
2 to changes in sources or sinks. to adjust
If we make changes now, it will still be many, many years before the effect will be felt!
Methane
Produced naturally in anaerobic processes (e.g., decomposition of plant material in swamps & bogs)
Has increased becaue of the following activities: raising cattle / livestock, rice, production, landfill decomposition, pipeline leaks
Has relatively short atmospheric residence time ( 10 years ) because it reacts with OH to form H
2
O and CO
2
.
Nitrous Oxide
Produced naturally in soils
Has increased because of fossil fuel combustion (esp. diesel), forest burning, use of nitrogen fertilizers
Has a long atmospheric residence time (~150 years)