Document
advertisement

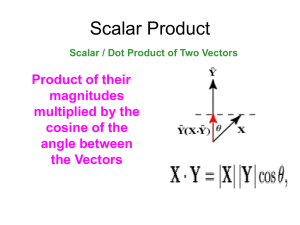
THERMODYNAMICS, TRANSPORT PROPERTIES AND KINETICS OF PARTIALLY IONIZED GASES M. Capitelli Chemistry Department, University of Bari, Italy IMIP-CNR, section of Bari, Italy Influence of Electronically Excited States on Thermodynamic Properties of LTE Hydrogen Plasma Internal partition function Fint calculated with nmax 1 a0 3 N where N is the particle density (cm3) N max 2n 2e n 1 En kT The total enthalpy of all the system is calculated as follows: D D 5 5 5 H n' H kT E n' H kT EI n' e kT 2 2 2 2 2 while the inner part of enthalpy is equal to: Hint n'H E Ratio of inner enthalpy to total enthalpy at different pressure. c p tot the total specific heat is diveded in two parts: H 5 5 5 cPf n ' k c n ' k n ' H Vint e k H T P ,n 'i 2 2 2 the frozen specific heat the reactive specific heat H c p f c p r T P c Pr D n' H n' H 5 kT E 2 T T P 2 the inner specific heat is : Ratio of inner Cp to Cp frozen at different pressure 5 n' D 5 kT EI e kT 2 2 T P 2 P cint n' H cV int Ratio of inner Cp to total Cp at different pressure Internal specific heat and his component at 105 Pa. Ratio of Cp, using Debye-Huckel theory, to Cp calculated with cut-off, at 105 Pa. Internal specific heat and his component at 108 Pa. Ratio of Cp, using Debye-Huckel theory, to Cp calculated with cut-off, at 108 Pa. Influence of Electronically Excited States on Transport Properties of LTE Hydrogen Plasma The transport properties of a partially ionized thermal hydrogen plasma has been calculated by taking into account electronically excited states with their “abnormal” transport cross sections. The results show a strong dependence of these transport properties on electronically excited states specially at high pressure. Heavy particles transport properties • • Translational thermal conductivity Viscosity • H n H H 1H H n H m H 1H 1 • • Translational thermal conductivity Electrical conductivity Reactive thermal conductivity “Usual” Collision integrals H n e H 1e Electron transport properties “Abnormal” Collision integrals Complete set of data (see text) Model • Species We have considered an hydrogen plasma constituted by molecular hydrogen, atomic hydrogen (12 atomic levels), H+ ions and electrons: • • • • H2 H(n=1,12) H+ e- • Reactions H 2 2H1 H n 1,12 H e we consider the dissociation process and ionization reactions starting from each electronic states of hydrogen atom. Equilibrium Composition The equilibrium composition is obtained by using Saha and Boltzmann laws. First we calculate the equilibrium composition by considering only four species and two reactions which take into account the total atomic hydrogen without distinction among the electronic states. 32 32 E D N H2 QH 2mekbT 1 exp 2 N H 2 QH 2 h 2 k bT Ne NH NH E I 2 gH 2m e k bT 3 2 exp QH h 2 k bT g ni n i eE i Z T kB T Then we use the Boltzmann distribution for calculating the distribution of the electronic states of atomic hydrogen. Collision Integrals I: General Aspects Considered interactions: Transport cross sections can be calculated as a function of gas temperature according to the equations • neutral-neutral (H2-H2, H2-H, H-H) • ion-neutral (H+-H2, H+-H) • electron-neutral (e-H, e-H2) • charged-charged (H+-e, H+-H+, e-e) ij(l,s) For the present calculation we need the collision integrals of different orders depending on the different approximations used in the Chapman-Enskog method. To this end we have used a recursive formula d (l,s ) 3 (l,s ) T s 2 dT 0 e ij2 ij2s3 (l ) (g)d ij (l ) (g) 2 0 (1 cosl )bdb (l,s 1) kT 2ij where 1 2 ij2 ij g 2 / KT Note that we have used the reduced collision integrals i.e. the collision integrals normalized to the rigid sphere model ij* Collision Integrals II: e-H(n) 250.0 Diffusion type collision integrals for the interactions e-H(n) are calculated by integrating momentum transfer cross sections of Ignjatovic* et al.. n=10 T=104 K 2 H(n)-e ( Å ) 200.0 150.0 100.0 T=2 104 K 50.0 n=1 0.0 1.0 1.2 1.4 1.6 n' = 2 - 1/n2 Viscosity type collision integrals have been considered equal to diffusion type ones eHn eHn 2,2 * *L.J. Ignjatovic, A.A. Mihajlov, Contribution to Plasma Physics 37 (1997) 309. 1,1 * 1.8 2.0 Collision Integrals III: H+-H(n) Diffusion type and viscosity type collision integrals have been calculated by Capitelli et al.* and fitted according to the following expressions. (2,2) 2 H(n)+H + (Å ) 10 3 10 2 10 f3 4 10 3 10 2 10 1 2 exp f 4 n f 5 n=5 n=4 n=3 n=2 n=1 1.0 T =104 K 1.2 1.4 1.6 1.8 2.0 n 2 n12 H nH T exp g1ng2T g3 exp g4 n g5 2,2 * n=5 n=4 n=3 1 n=2 0 1.0 1.2 1.4 1.6 1 n' = 2 - 2 n 1.8 with n=1 10 10 (1,1) T exp f1n T f2 5 T =104 K H(n)-H + (Å ) 1,1 * H nH ct 10 2.0 T T 1000 The elastic contribution to (1,1)* has been evaluated with a polarizability model *M. Capitelli, U.T. Lamanna, J. Plasma Phys.12, 71 (1974). Collision Integrals IV: H(n)-H(n) Viscosity type collision integrals for the interactions H(n)-H(n) up to n=5 have been calculated by Celiberto et al.* By using potential energy curves obtained by CI (configuration interaction) method. The data have been interpolated at different temperatures with the equation H nH n exp a1 exp a2n a3 2,2 * where n 2 1 n2 30.0 T=104 K H(n)-H(n) 2 (Å ) 25.0 20.0 15.0 10.0 5.0 0.0 T=2 104 K n=1 1.0 1.2 1.4 1.6 n'= 2 - 1/n2 *R. n=5 Celiberto, U.T. Lamanna, M.Capitelli, Phys.Rev A 58, 2106 (1998). 1.8 2.0 10 6 10 5 10 4 10 3 10 2 10 1 10 0 10 -1 10 -2 m=n+1 4 T =10 K M.Capitelli, P.Celiberto, C.Gorse, A.Laricchiuta, P.Minelli, D.Pagano, Phys. Rev. E 66,016403/1 (2002) m=n+2 (3,4) m=n+3 (2,3) (2,4) (1,2) (1,3) n=4 n=3 n=2 n=1 1.0 1.2 1.4 1.6 1.8 30.0 2.0 25.0 2 1 T H2,2n*H n H2,2m*H m 2 (2,2) 2,2 * H nH m 4 T =10 K n 2 12 n H(n)-H(m) (Å ) (1,1) H(n)-H(m) 2 (Å ) Collision Integrals V: H(n)-H(m) m=2 m=1 (3,3) 15.0 10.0 5.0 m=3 (4,4) 20.0 (2,2) (1,1) n=4 n=3 n=2 0.0 n=1 1.0 1.2 1.4 1.6 1 n' = 2 - 2 n 1.8 2.0 Transport Coefficients I: General Aspects Transport coefficients have been calculated by using the third approximation of the Chapman-Enskog method for the electron component and the first non-vanishing approximation for heavy components. Cut-off criterium In general we have considered 12 electronically excited states; at high pressure we have reduced the number of excited states to 7 to take into account the decrease of the number of the electronically excited states with increasing pressure. We include in the electronic partition function all the elctronic states with radius less than the average distance between particles 1 2 a0 nmax where 1 3 n n p kBT Transport Coefficients II: Translational Thermal Conductivity Heavy particles Electrons Chapman-Enskog method Second order approximation L11 Third order approximation L1v x1 1 htr 4 Lv1 Lvv x v x1 L11 xv 0 L1v 2k BTe 2 75 16 q 22 e tr 10 nek B 2 8 me q 11q 22 q 12 Lii Lv1 Lvv Lij A * ij 4 xi 2 v ii 2xj xi ij K 1 K i 2x i x K iK M i M j 2 MiMj M i M j 2)* (2, ij (1ij,1)* 1 2 B * ij 1 15 2 25 2 * * M i M K 3M K2 BiK 4 M i M K AiK * AiK 2 4 1 55 3Bij* 4Aij* * AiK 4 5(1ij, 2)* 4(1ij, 3)* (1ij,1)* Transport Coefficients III: Reactive Thermal Conductivity For a gas constituted by chemical species and independent reactions the reactive thermal conductivity can be calculated by means of Butler and Brokaw theory where v1 Aij k1 aik ail a jk a jl RT x x D P K l x x x x kl k l k l lk 1 v R A11 A1 H 1 A 1 A H 1 H 1 RT 2 A11 H 0 A1 A 1 A Transport Coefficients IV: Viscosity Viscosity has been calculated by means of the first approximation of the Chapman-Enskog method The Hij are expressed as a function of temperature, collision integrals and molecular weight of the species, while i represents the molar fraction of the i-th component H 11 H 1v x1 H v1 H vv x v x1 H 11 xv 0 H 1v H v1 H vv 2x x M i M k 5 M k i k H ii * 2 ii k1 3A M M M ik i ik i k xi2 v ki 2x x M i M j 5 i j H ij 1 * ij M M 2 3A ik i j A * ij )* (2,2 ij (1,1)* ij ij 2.6693 2 2M i M j P0 Dij 10 2.628 M i M j Aij*T Transport Coefficients V: Electrical Conductivity The electrical conductivity has been calculated by using the third approximation of the Chapman-Enskog method 2 q 11q 22 q 12 3 2 2 2 e ne 2 2 me KT q 00 q 11q 22 q 12 q 01 q 12q 02 q 01q 22 q 02 q 01q 12 q 02q 11 2 1 where 1 00 q 8n e n j Q ej(1,1)* q 8 2 ne Q 11 j 1 5 8n e n j Q ej(1,1)* 3Q ej(1,2 )* 2 j 1 1 q 01 q 12 v 1 175 7 (2,2 )* (2,3)* 8 2 n e Q ee 2Q ee 8 n j Q ej(1,1)* 4 j 1 16 35 21 8n e n j Q ej(1,1)* Q ej(1,2 )* 6Q ej(1,3)* 8 2 j 1 1 q 02 q The presence of electronically excited states can affect e through the collisions e-H(n) 25 8 n j Q ej(1,1)* 15Q ej(1,2 )* 12Q ej(1,3)* 4 j 1 v 1 (2,2 )* ee 22 315 (1,2 )* Q ej 57Q ej(1,3)* 30Q ej(1,4 )* 8 v 1 1225 (1,1)* 77 (2,2 )* (2,3)* (2,4 )* 8 2 n e Q ee 7Q ee 5Q ee 8 n j Q ej 16 j 1 64 735 (1,2 )* 399 (1,3)* Q ej Q ej 210Q ej(1,4 )* 90Q ej(1,5 )* 8 2 10 8.0 10 -5 8 6.0 10 -5 6 4.0 10 -5 4 2.0 10 -5 2 -1 Including off-diagonal terms 1.5 10 2 10 2.5 10 Temperature [K] 4 “usual” collision integrals: solid line “abnormal” collision integrals: dashed line 04 3 10 -5 4.0 10 -5 -1 80 60 40 -5 (b) 2.0 10 20 0 0.0 10 4 1 10 (a) This point can indicate the importance of using higher Chapman - Enskog approximations for the calculation of the viscosity in the presence of excited states. 6.0 10 100 (b) The differences calculated with the two sets of collision integrals are very higher. -5 | - |*100 / Neglecting off-diagonal terms 8.0 10 -1 4 The small relative error indicate a sort of compensation between diagonal and offdiagonal terms. (b) 4 (a) 0 0.0 10 4 1 10 Viscosity [Kg m s ] -1 -4 (b) 1.0 10 | - |*100 / Viscosity [Kg m s ] Results I: Diagonal Approximation (Viscosity) 1.5 10 4 2 10 Temperature [K] 4 0 4 2.5 10 Results II: Heavy Particles Translational Thermal Conductivity The ratio between the translational thermal conductivity values calculated with the “abnormal” cross sections (ha) and the corresponding results calculated with the “usual” cross sections (hu) is reported as a function of temperature for different pressures. 4.00 100atm 3.00 2.00 (a) h [W m-1 K-1] 5.00 n=7 10atm 1.00 0.00 10000 n=12 1atm 1.00 15000 20000 25000 1atm 30000 0.90 The small effect observed at 1 atm is due to the compensation effect between diagonal and offdiagonal terms in the whole representation of the translational thermal conductivity of the heavy components. This compensation disappears at high pressure as a result of the shifting of the ionization equilibrium (a)h / (u) h Temperature [K] 0.80 10atm 0.70 n=7 0.60 100atm 0.50 0.40 0.30 10000 n=12 15000 20000 25000 Temperature [K] 30000 Results III: Electron Translational Thermal Conductivity 12.00 100atm 8.00 n=7 n=12 6.00 10atm 4.00 1atm The figure reports the ratio ea/ eu calculated with the two sets of collision integrals as a function of temperature at different pressures. 2.00 0.00 10000 1.00 15000 20000 25000 1atm 30000 10atm Temperature [K] Again we observe that the excited states increase their influence with increasing the pressure. At high pressure the deviation decreases when considering only 7 excited states. (u) (a) e / e (a)e [W m-1 K-1] 10.00 In this case the presence of excited states affects only the interactions of electrons with H(n). 0.95 n=7 0.90 100atm 0.85 n=12 0.80 10000 15000 20000 25000 Temperature [K] 30000 Results IV: Reactive Thermal Conductivity 6.00 This contribution has been extensively analyzed in a previous paper*. (a) r [W m-1 K-1] 1atm 10atm n=12 4.00 n=7 100atm The main conclusions follow the illustrated for h and e in this work. trend 2.00 0.00 10000 15000 20000 25000 1.00 1atm Temperature [K] (a)r / (u) r 0.90 0.80 10atm 0.70 n=7 0.60 100atm 0.50 n=12 *M.Capitelli, P.Celiberto, C.Gorse, A.Laricchiuta, P.Minelli, D.Pagano, Phys. Rev. E 66,016403/1 (2002) 0.40 10000 15000 20000 Temperature [K] 25000 Results V: Viscosity [Kg m-1 s-1] 1.0 10-4 (a) 1.5 10-4 5.0 10-5 The results for viscosity are in line with those discussed for the heavy particles translational contribution to the total thermal conductivity. 100atm n=7 n=12 10atm 1atm 0.0 100 10000 15000 20000 25000 30000 Temperature [K] (a) / (u) The viscosity values calculated with the “abnormal” cross sections ((a)) are less than the corresponding results calculated with the “usual” cross sections ((u)). The relative error decreases when, at high pressure, seven excited states are included in the calculation. 1.00 1atm 0.80 10atm n=7 0.60 100atm 0.40 n=12 0.20 10000 15000 20000 25000 Temperature [K] 30000 Results VI: Electrical Conductivity 3.0 104 2.0 104 10atm 100atm n=12 1atm 1.0 104 0.0 100 10000 15000 20000 25000 30000 1atm 1.00 Temperature[K] 10atm 0.95 (u) (a) e / e (a) e [S m-1] 100atm n=7 The trend of the electrical conductivity follows that one described for the contribution of electrons to the total thermal conductivity. 0.90 n=7 0.85 100atm 0.80 0.75 n=12 0.70 0.65 10000 15000 20000 25000 Temperature[K] 30000 Results VII: Number of levels in partition function 1.00 n=1 n=2 n=1 1.00 n=2 (u) (a) e / e 0.80 n=4 0.70 0.60 0.95 n=4 0.90 0.85 0.50 (a) 0.40 10000 n=6 n=6 (b) 15000 20000 25000 0.80 10000 30000 15000 Temperature [K] 20000 1.00 Figures show the ratio between transport coefficients calculated by using “abnormal” and “usual” collision integrals at pressure of 1000 atm, as a function of temperature and for different number of atomic levels. 25000 30000 Temperature [K] n=1 0.90 n=2 (u) (a) r / r (a) / (u)h h 0.90 0.80 n=4 0.70 n=6 0.60 0.50 (c) 0.40 10000 15000 20000 Temperature [K] 25000 30000 1.00 At high pressure the number of excited states decrease. However increasing pressure, the ionization equilibrium is shifted to higher temperatures so that the concentration of low lying excited states can be sufficient to affect the transport properties n=1 n=2 0.80 n=4 0.70 0.60 0.50 n=6 (e) 0.40 10000 15000 20000 25000 30000 Temperature [K] 1.00 n=1 n=2 0.95 We can see that in this case already the first excited state (n=2) affects the results. (u) (a) e / e (a) / (u) 0.90 n=4 0.90 0.85 0.80 (f) 0.75 10000 n=6 15000 20000 25000 Temperature[K] 30000 Conclusions The results reported indicate a strong dependence of the transport properties of LTE H2 plasmas on the presence of electronically excited states. This conclusion is reached when comparing the transport coefficients calculated with the two sets of collisison integrals. Our results emphasize the importance of these states in affecting the transport coefficients specially at high pressure. Another point to be discussed is the accuracy of the present calculations with respect to the Chapman-Enskog approximation used in the present work. But at high pressure a question is open concerning the number of excited states to be included in the calculation of the partition function. These approximations are very accurate when neglecting the presence of excited states. In the presence of excited states with their “abnormal” transport cross sections these approximations could not be sufficient. Collisional-Radiative Model for Atomic Plasma 1. Excitation and de-excitation by electron impact k ij A(i) e () A( j) e () k ji 2. Ionization by electron impact and three body recombination ic k eb ( b ) A(i) e () A e () k ci 3. Spontaneous emission and absorption A ij ij A(i) A( j) h ij 4. with i > j Radiative recombination i A e () A(i) h Rate Equations dn i n jA*ji ne n jk ji n2e n k ci ne ni n i A*ij n i ne k ij n i ne k ic dt ji ji ji ji i dn e dn dn i dt dt i dt Stationary solution dn i 0 dt i Quasi-Stationary Solution(QSS) dn i 0 i 2 dt dn1 dn dn e dt dt dt Time-dependent solution dn i 0 dt i QSS approximation dn i 0 i 2 dt dn1 dn e dn dt dt dt The ground state density changes like the density of the charged particles and the excited states are in an instantaneous ionization-recombination equilibrium with the free electrons differential equation for the ground state * Xi i dX1 a11X1 a1jX j b1 dt j2 ni nSB i system of linear equation for excited levels i* a ij X j b i i 1 j1 Xj (j>1) can be calculated when X1, ne, Te are given The system of equations is linear in X1 X i2 X i2 f (X1 , ne ,Te ) X0i2 R1i2 X1 X0i2 f (n e ,Te ) R1i2 f (n e ,Te ) CR for Atomic Nitrogen Plasma: Energy-level Model gro up Energy (cm-1) Statistical weight 1 0 4 2 19228 10 3 28840 6 4 83337 12 5 86193 6 6 95276 36 7 96793 18 8 103862 18 9 104857 60 10 104902 30 11 107125 54 12 109951 18 13 110315 90 14 110486 126 15 111363 54 16 112691 18 17 112851 90 18 112955 288 19 113391 54 20 114211 90 21 114255 486 22 114914 882 23 115464 1152 24 115837 1458 25 116102 1800 26 116298 2178 27 116445 2592 28 116560 3042 29 116650 3528 30 116724 4050 31 116784 4608 32 116834 5202 33 116875 5832 34 116910 6498 35 116940 7200 Terms 2p34S 2p32D 2p32P 3s4P 3s”P 3p4S, 4P, 4D 3p2S,2P,2D 4s4P,2P 3d4P,4D,4F 3d2P,2D,2F 4p4S,4P,4D,2S,2D,2P 5s4P,2P 4d4P,4D,4F,2P,2D,2F 4f4D,4F,4G,2D,2F,2G 5p4S,4P,4D,2S,2P,2D 6s4P,2P 5d4P,4D,4F,2P,2D,2F 5f,5g 6p 6d4P,4D,4F,2P,2D,2F 6f,6g,6h n=7 n=8 n=9 n=10 n=11 n=12 n=13 n=14 n=15 n=16 n=17 n=18 n=19 n=20 CR for Atomic Nitrogen Plasma with QSS Xi vs level energy Te=5800 K Te=11600 K 10 10 1 1 0.1 0.1 Te=17400 K 100 10 i X i X i 1 X 0.1 0.01 X =1 X =1 0.01 X =1 1 1 1 0.01 8 n =10 cm 0.001 16 ne=10 cm -1 level energy (cm ) 16 n =10 cm -3 0.0001 4 4 4 4 5 5 5 5 5 8 10 8.5 10 9 10 9.5 10 1 10 1.05 10 1.1 10 1.15 10 1.2 10 e e 0.001 e n =108 cm -3 n =108 cm -3 -3 -3 e 0.0001 4 4 4 4 5 5 5 5 5 8 10 8.5 10 9 10 9.5 10 1 10 1.05 10 1.1 10 1.15 10 1.2 10 -1 level energy (cm ) ne=1016 cm -3 0.001 0.0001 4 4 4 4 5 5 5 5 5 8 10 8.5 10 9 10 9.5 10 1 10 1.05 10 1.1 10 1.15 10 1.2 10 -1 level energy (cm ) Time-dependent solution dn i 0 dt i Rate coefficients for electron impact processes k f ()()v()d Et f() electron energy distribution function () cross section v() electron velocity rate coefficients CR rate equations f() Boltzmann equation level population plasma composition H(n 25), H ,e Atomic Hydrogen Plasma H+ = e- =10-8 , H=1, H(1)=1, H(i)=0 P=100 Torr, Tg=30000 K, Te(t=0)=1000 K Xi = ni/niSB vs time(s) density (cm-3) vs time(s) i>1 Te vs time(s) 35000 4 10 16 10 100 30000 1 25000 0.01 0.0001 -3 20000 -6 10 1012 H 15000 -10 10 + H e -8 10 e X i T (K) density (cm ) 1014 i=1 i=2 i=5 i=10 i=15 i=20 i=25 -12 10 - -14 10 10 10 -16 10 10000 5000 -18 10 108 10-5 -20 10 10-4 10-3 time (s) 10-2 10-1 -9 10 -8 10 -7 10 -6 10 -5 10 time (s) -4 10 -3 10 -2 10 -1 10 0 -14 -12 -10 -8 10 10 10 10 -6 10 -4 10 time (s) -2 10 0 10 2 10 4 10 6 10 H(i)/g(i) vs Ei eedf(eV-3/2) vs E t(s)=0 s -10 t(s)=10 T = 29986 K 0.01 fit 0.0001 10 1 -9 0.01 t(s)=10 s -8 -10 -14 t(s)=8 10 s -16 t(s)=10 s -8 10 -7 10 -18 -6 10 t(s)=10 s -20 10 -5 t(s)=10 s -22 10 -4 -24 -4 -30 t(s)=10 s -3 0 2 4 6 8 level energy (eV) 10 12 14 -12 t(s)=5 10 s -14 -16 t(s)=8 10 s -7 t(s)=10 s -18 t(s)=10 s -20 t(s)=10 s -22 t(s)=10 s -24 t(s)=8 10 s -26 t(s)=9 10 s -8 10 -8 10 10 -6 10 -5 10 10 t(s)=9 10 s 10 t(s)=3 10 s -4 -28 10 -8 -10 10 t(s)=8 10 s -26 10 t(s)=10 s 10 t(s)=10 s 10 -8 -8 -3/2 t(s)=5 10 s s -9 t(s)=10 s -9 t(s)=5 10 s 10 eedf (eV ) -12 -10 t(s)=10 -6 -8 10 fit 10 -8 t(s)=3 10 s 10 t(s)=0 s T = 30020 K 0.0001 -8 10 H t(s)=10 s t(s)=5 10 s -6 (i)/g(i) s -9 -4 -4 -4 10 -3 t(s)=10 s -28 10 -30 10 0 5 10 15 (eV) 20 25 Non-Equilibrium Kinetics in High Enthalpy Nozzle Flows exit reservoir throat coupling state-to-state kinetics with fluid dynamic models - Numerical aspects - Coupling with kinetics - Chemical kinetics - Vibrational kinetics - Metastable state kinetics - Boltzmann equation - Coupling with chemical kinetics - EM fields contribution