Nuclear Reactions
advertisement

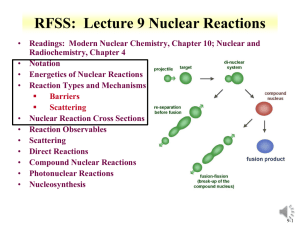
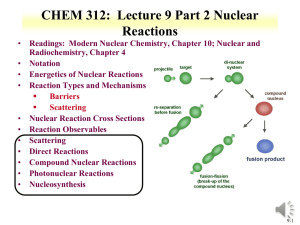
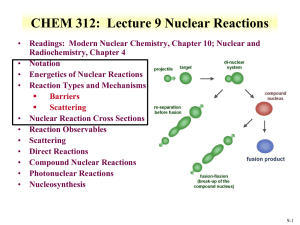
RDCH 702: Lecture 3, Nuclear Reactions • Readings: Modern Nuclear Chemistry, Chapter 10; Nuclear and Radiochemistry, Chapter 4 • Notation • Energetics of Nuclear Reactions • Reaction Types and Mechanisms Barriers Scattering • Nuclear Reaction Cross Sections • Reaction Observables • Scattering • Direct Reactions • Compound Nuclear Reactions • Photonuclear Reactions • Nucleosynthesis 3-1 Nuclear Reactions • • Nucleus reactions with a range of particles Nucleus, subatomic particle, or photon to produce other nuclei Short time frame (picosecond) Energetics involved in reaction First nuclear reaction from Rutherford What reaction was this? Number of terms conserved during nuclear reactions Basis for understanding and evaluating reactions Number of nucleons * except in reactions involving creation or annihilation of antinucleons Charge Energy/Mass Momentum Angular momentum Parity Q is the energy of the reaction • positive Q corresponds to energy release negative Q to energy absorption Q terms given per nucleus transformed • • 14 7 N He O H Q 4 2 Shorthand: 17 8 14 1 1 N ( , p) O 17 3-2 Energetics • Energetically many orders of magnitude greater than chemical reactions • 14N(,p)17 O; Q=-1.193 MeV Convert energy to per molar basis 1 eV = 1.60E-19 J =-7.18E23 MeV/mole= -7.18E29 eV/mole =-1.15E11 J/mole • Reactions so large that mass change is observable • Q value can be experimentally measured to provide a route to determine particle mass of reactants Mass and energy balance Know Q value, determine unknown mass 3-3 Energetics • Reaction Q values Not necessarily equal to kinetic energy of bombarding particles for the reaction to occur Need more energy than Q value for reaction to occur * Reaction products will have kinetic energy that needs to come from reaction • Conservation of momentum Some particles’ kinetic energy must be retained by products as kinetic energy • Amount retained as kinetic energy of products Based on projectile mass Retained kinetic energy becomes smaller with increasing target mass APr ojectile Equation for kinetic energy (T): T Q • What does this mean about reaction APr ojectile AT arg et Heavier target or heavier projectile? 248Cm + 18O266Rf 18 248 T Q 0.932Q 248 18 T 248Cm Projectile 248 18 18O Q 0.068Q Projectile 3-4 Energetics: Reaction Barrier • • Need to consider laboratory and center of mass frame Laboratory frame conservation of momentum considers angle of particles mp mx 2 Q Tx (1 ) Tp (1 ) (m pTp mxTx ) cosq mR mR mR • • • • Q value can be found if Tx and q are measured and particles known Tp from experiment Center of mass Total particle angular momentum is zero v p mp 2 (m p mT )vcm vcm Tcm (m p mT ) 2 Kinetic energy carried by projectile (Tlab) is not fully available for reaction Tlab - Tcm = T0 T0 is energy to be dissipated in reaction For reaction to occur Q + T0 must be achieved Basis for threshold reaction Q + T0 > 0 Q Tx Tp TR Tcm Tlab ( mp m p mT ) 3-5 • Reaction Barrier Threshold energy (minimum energy for reaction) Q Tlab TCM 0; Tcm Tlab ( Tlab Tlab ( Tlab (1 ( Tlab m p mT mp m p mT m p mT ) Solve of laboratory T ) Q )) Q Q Q Q mp m p mT mp mT (1 ( )) ( ( )) m p mT m p mT m p mT m p mT T Q • mp mp APr ojectile AT arg et AT arg et A for mass MeV Fraction of bombarding particle’s kinetic energy retained as kinetic energy of products becomes smaller with increasing mass of target Heavier target or heavier projectile? 248Cm + 18O266Rf 3-6 Reaction Barrier: Threshold Energy • Consider the 14N(,p)17O reaction A AT arg et Find threshold energy T Q Pr ojectile MeV Q from mass excess AT arg et * Q=2.425 + 2.863 – 7.289 – (-0.809) = -1.19 MeV T ( )1.19 • • • • 4 14 MeV 1.53MeV 14 Reaction barrier also induced by Coulomb interaction Need to have enough energy to react and overcome Coulomb barrier From charge repulse as particle approach each other * R is radius Z1 Z 2 e 2 Vc R ro A1 / 3 * ro =1.1 to 1.6 fm R1 R2 Equation can vary due to ro Z1 Z 2 Vc can be above threshold energy Vc 0.96 1 / 3 MeV 1/ 3 2*7 Vc 0.96 1 / 3 MeV 3.36 MeV 1/ 3 4 14 A1 A2 Center of mass, need to bring to laboratory frame Consider kinetic energy carried by projectile 3.36x ((14+4)/14) = 4.32 MeV alpha needed for reaction 3-7 Equations for production reactions: Cross Sections • Probability of a nuclear process is generally expressed in terms of a cross section dimensions of an area • Originates from probability for reaction between nucleus and impinging particle is proportional to the cross-sectional target area presented by the nucleus Doesn’t hold for charged particles that have to overcome Coulomb barriers or for slow neutrons • Total cross section for collision with fast particle is never greater than twice the geometrical crosssectional area of the nucleus cross section is close to 1 barn for this case • 10-24 cm2=1 barn 3-8 Cross sections • Accelerator: beam of particles striking a thin target with minimum beam attenuation Ri Inx i • When a sample is embedded in a uniform flux of particles incident on it from all direction, such as in a nuclear reactor, the cross section is defined: Ri N i Ri= # of processes of type under consideration occurring in the target per unit time I= # of incident particles per unit time n= # of nuclei/cm3 x=target thickness (cm) =flux of particles/cm2/sec N=number of nuclei contained in sample 3-9 Production of radionuclides N 0 =cross section N1 (1 e l1t ) l1 =neutron flux l1t N l A N ( 1 e ) t=time of irradiation 1 1 1 0 (1-e-(lt)) * maximum level (saturation factor) • Activity of radioactive product at end bombardment is divided by saturation factor, formation rate is obtained R=A/(1-e-(lt)) half life % 1 50 2 75 3 87.5 4 93.75 5 96.875 3-10 Nuclei production: Short irradiation compared to half-life • Find amount of 59Fe (t1/2=44.5 d, l = 1.803E-7 s-1) from irradiation of 1 g of Fe in a neutron flux of 1E13 n/cm2/s for 1 hour 58Fe(n,g)59Fe: 58Fe+ n g + 59Fe 1.3E-24 cm2 No= 1g/55.845 g/mol *6.02E23 atom/mol*0.00282 No=3.04E19 atom • R= 1E13 n/cm2/s *1.3E-24 cm2 * 3.04E21 atom • R=3.952E8 atoms/sec Ri N i • 1.423E12 atoms 59Fe in 1 hour 3.04E19(1.3E - 24)(1E13) N1 (1 e 1.803E-7*3600 ) 1.803E- 7 N 0 3.952E8 N1 (1 e l1t ) N1 (1 9.994E 1) l1 1.803E- 7 N1 2.192E15(6.489E 4) 1.422E12 atom s 3-11 Nuclei production: Long irradiation compared to half-life • Find amount of 56Mn (t1/2=2.578 hr, l = 7.469E-5 s-1) from irradiation of 1 g of Mn in a neutron flux of 1E13 n/cm2/s for 1 hour 55Mn(n,g)56Mn: 55Mn+ n g + 56Mn 13.3E-24 cm2 No= 1g/54.93804 g/mol *6.02E23 atom/mol No=1.096E22 atom • R= 1E13 n/cm2/s *13.3E-24 cm2 * 1.096E22 atom Ri N i • R=1.457E12 atoms/sec • 5.247E15 atoms 56Mn in 1 hour (does not account for decay) N 0 l1t 1.096e22( 13.3E- 24)(1E13) 7.469E -5*3600 N ( 1 e ) N1 (1 e ) 1 l1 7.469E- 5 1.458E12 N1 (1 7.642E 1) 7.469E- 5 N1 1.952E16(2.358E 1) 4.603E15 atom s 3-12 Formation rate from activity • R=A/(1-e-(lt)) • 4.603E15 atoms 56Mn (t1/2=2.578 hr, l = 7.469E5 s-1) from 1 hour irradiation • A=lN= 4.603E15* 7.469E-5 =3.436E11 Bq • R=A/(1-e-(lt)) • R= 3.436E11/(1-exp(- 7.469E-5 *3600)) • R=1.457E12 atom/sec 3-13 Cross Section Values and Limits • Reaction cross section of R2 is approximated at high energies Wave nature of incident particle causes upper limit of reaction cross section to include de Broglie wavelength So cross section can be larger than area due to incoming particle wavelength Expressed as an increase in R, quantum in nature r ( R ) 2 • Collision between neutron and target nucleus characterized by distance of closest approach B is impact parameter 3-14 Cross sections • Angular momentum of system is normal to the relative momentum p b L pb l b l • b any value between 0 and R l b (l 1) • l =0,1,2,…b angular momentum 2 r ( R ) lħ • Sum all l from 0 to lmax • Cross section based on summation of l cross sections • For this reason nuclear reaction cross sections can be several orders of magnitude larger than the nuclear geometrical cross section Manifest by slow-neutron reactions 3-15 Cross section l is partial cross section of given angular momentum l l [(l 1) l ] (2l 1) 2 • 2 2 2 Quantum-mechanical treatment Tl is the transmission coefficient for reaction of a neutron with angular momentum l Represents fraction of incident particles with angular momentum l that penetrate within range of nuclear forces Provides summing term to increase cross section Reason why cross section can be larger than physical size of nucleus r 2 (2l 1T l 0 l • General trends for neutron and charged particles Charged particle cross section minimal at low energy Neutron capture cross section maximum at low energy 3-16 • • Measuring Cross Section: Excitation Functions Variation of reaction cross section with incident energy Shape can be determined by exposing several target foils in same beam with energydegrading Simultaneous measurement of multiple particle energies • Provide information about probabilities for emission of various kinds and combination of particles in nuclear reactions formation of given product implies what particles were ejected from target nuclide • Range of cross sections can be evaluated Detection limit of product can influence cross section limit measurement 3-17 Barriers for Charged Particles • Coulomb repulsion between charged bombarding particles and nucleus Repulsion increases with decreasing distance of separation until charged particle comes within range of nuclear forces Probability of tunneling through barrier drops rapidly as energy of particle decreases Coulomb barriers affect charged particles both entering and leaving the nucleus Charged particles emitted from nuclei experience Coulomb repulsion during emission greater than 1 MeV seen with position emission • Related to change in cross section with energy for charged particle reactions Maximum cross section dependent upon energy 3-18 RDCH 702: Lecture 3, Nuclear Reactions • Readings: Modern Nuclear Chemistry, Chapter 10; Nuclear and Radiochemistry, Chapter 4 • Notation • Energetics of Nuclear Reactions • Reaction Types and Mechanisms Barriers Scattering • Nuclear Reaction Cross Sections • Reaction Observables • Scattering • Direct Reactions • Compound Nuclear Reactions • Photonuclear Reactions • Nucleosynthesis 3-19 Reactions: Elastic Scattering • Elastic scattering kinetic energy conserved Particles do not change • Simplest consequence of a nuclear collision Not a “reaction” no exchange of nucleons or creation of particles • Particles do not change their identity during the process and the sum of their kinetic energies remains constant • Elastic scattering will also have a contribution from nuclear forces 3-20 • Low-Energy Reactions with Light Slow-Neutron Reactions Projectiles Purest example of compound-nucleus behavior 1/v law governs most neutron cross sections in region of thermal energies neutrons available only from nuclear reactions Range of energies can be obtained • Reaction Cross Sections Coulomb barrier prevents study of nuclear reactions with charged particles below 1 MeV resonances no longer observable with increasing energy, increasing variety of reactions possible 3-21 Low-Energy Reactions • Deuteron Reactions Prevalence of one nucleon stripping large size and loose binding of deuteron Only proton and neutron in deuteron nucleus * Proton charge carries both nucleons Neutron comes within range of nuclear forces while proton is still outside most of Coulomb barrier Inherent in large neutron-proton distance in deuteron weakly bound deuteron can be broken up * proton outside barrier • Competition among Reactions depends on relative probabilities for emission of various particles from compound nucleus determined by number of factors * energy available * Coulomb barrier * density of final states in product nucleus 3-22 High Energy Reactions • • Spallation Products products in immediate neighborhood of target element found in highest yields within 10 to 20 mass numbers yields tend to form in two regions stability for medium-weight products neutron-deficient side of stability with increasing Z of products Used to produce beam of neutrons at spallation neutron source Heavy Z will produce 20-30 neutrons Basis of Spallation neutron source (http://neutrons.ornl.gov/facilities/SNS/) High-Energy Fission single broad peak in mass-yield curve instead of double hump seen in thermal-neutron fission many neutron-deficient nuclides especially among heavy products originate from processes involving higher deposition energies lower kinetic energies do not appear to have partners of comparable mass arise from spallation-like or fragmentation reactions 3-23 High-Energy Reactions • • Mass-Yield Curves at low energies, compound-nucleus picture dominates as energy increases importance of direct reactions and preequilibrium (pre-compound nucleus) emission increase above 100 MeV, nuclear reactions proceed nearly completely by direct interactions products down to mass number 150 are spallation products those between mass numbers 60 and 140 are fission products Cascade-Evaporation Model Above 100 MeV reactions energy of the incident proton larger than interaction energy between the nucleons in the nucleus Wavelength less than average distance between nucleons proton will collide with one nucleon at a time within the nucleus * high-energy proton makes only a few collisions in nucleus * Produces nucleons with high energy 3-24 Heavy-Ion Reactions • Range of heavy ion reactions elastic and inelastic scattering compound-nucleus formation, direct interactions deeply inelastic reaction • Reactions influence by parameter impact parameter of collision kinetic energy of projectile 1/ 3 1/ 3 R ro A1 A2 masses of target projectile nuclei • Elastic and Inelastic Scattering, Coulomb Excitation elastic-scattering measurements used to obtain information on interaction radii R=R1+R2 between mass numbers A1 and A2 ( 3-25 Heavy Ion Reactions • Inelastic scattering scattering in which some of projectile’s kinetic energy transformed into excitation of target nucleus greatest importance at large impact parameters heavy ions valuable can excite high-spin states in target nuclei because of large angular momenta • Can experience Coulomb excitation high charges below Coulomb barrier heights and excite nuclei by purely electromagnetic interactions • Transfer Reactions stripping and pickup reactions prevalent with heavy ions take place at impact parameters just below those at which interactions are purely Coulombic angular distributions show oscillatory, diffraction-like pattern when transfer reaction to single, well-defined state observed 3-26 Heavy Ion Reactions: Deep Inelastic Reactions • Relatively large amounts of nuclear matter transferred between target and projectile Show strongly forward-peaked angular distributions “Grazing contact mechanism” • Products with masses in vicinity of projectile mass appear at angles other than classical grazing angle Relatively small kinetic energies • Total kinetic energies of products strongly correlated with amount of mass transfer Increasing mass difference of product and projectile lowers kinetic energy • Product will dissociate into two fragments Appreciable fraction of incident kinetic energy dissipated and goes into internal excitation 3-27 Compound-Nucleus Reactions • Compound-nucleus formation can only take place over a restricted range of small impact parameters can define critical angular momentum above which complete fusion cannot occur cf/R decreases with increasing bombarding energy • Neutron deficient heavy ions produce compound nuclei on neutron-deficient side of stability belt • Heavy ion of energy above Coulomb barrier brings enough excitation energy to evaporate several nucleons 5-10 MeV deexcitation for neutron evaporation • heavy-ion reactions needed for reaching predicted island of stability around Z=114 to N=184 • U is excitation energy, MA and Ma masses of target and projectile, Ta is projectile kinetic energy, Sa is projectile binding energy in compound nucleus MA U Ta S a M A Ma 3-28 3-29 Photonuclear reactions • Reactions between nuclei and lowand medium-energy photons dominated by giant resonance Excitation function for photon absorption goes through a broad maximum a few MeV wide Due to excitation of dipole vibrations of protons against neutrons in the nucleus • Resonance peak varies smoothly with A 24 MeV at 16O 13 MeV at 209Bi • Peak cross sections are 100-300 mb • (g, p), (g, n), (g,) reactions http://www.engin.umich.edu/research /cuos/ResearchGroups/HFS/Research /photonuclear_reactions.html 3-30 Natural Element Production • • • • • • Nuclear Astrophysics fundamental information on the properties of nuclei and their reactions to the perceived properties of astrological objects processes that occur in space Nuclear reactions responsible for production of elements Occurs in stars At temperatures and densities light elements are ionized and have high enough thermal velocities to induce a nuclear reaction heavier elements were created by a variety of nuclear processes in massive stellar systems systems must explode to disperse the heavy elements distribution of isotopes here on earth underlying information on the elemental abundances nuclear processes to produce the primordial elements 3-31 •Chart of the nuclide trends •Actinides some distance from stable elements 3-32 Timeline • Big bang 15E9 years ago • Temperature 1E9 K • Upon cooling influence of forces felt 2 hours H (89 %) and He (11 %) Free neutrons decay 3-33 Origin of Elements • • • • Gravitational coalescence of H and He into clouds Increase in temperature to fusion Proton reaction 1H + n → 2H + g 2H + 1H → 3He 2H + n → 3H 3H + 1H → 4He + g 3He + n → 4He + g 3H + 2H → 4He + n 2H + 2H → 4He + g 4He + 3H → 7Li + g 3He+4He → 7Be + g 7Be short lived Initial nucleosynthesis lasted 30 minutes * Consider neutron reaction and free neutron half life Further nucleosynthesis in stars No EC process in stars 3-34 Stellar Nucleosynthesis • He burning 4He + 4He ↔ 8Be + γ - 91.78 keV Too short lived 3 4He → 12C + γ + 7.367 MeV 12C + 4He →16O 16O + 4He →20Ne • Formation of 12C based on Hoyle state Excited nuclear state Somewhat different from ground state 12C Around 7.6 MeV above ground state 0+ Fusion up to Fe From binding energy curve Maximum at Fe • 3-35 Stellar Nucleosynthesis • CNO cycle 12C + 1H →13N + g 13N →13C + e++ νe 13C + 1H →14N + γ 14N + 1H →15O + γ 15O →15N + e+ + νe 15N + 1H →12C + 4He Net result is conversion of 4 protons to alpha particle 4 1H → 4He +2 e++ 2 νe +3 γ 3-36 Formation of elements A>60 Neutron Capture; S-process A>60 68Zn(n, γ) 69Zn, 69Zn → 69Ga + n mean times of neutron capture reactions longer than beta decay half-life Isotope can beta decay before another capture Up to Bi 3-37 Nucleosynthesis: R process • Neutron capture time scale very much less than - decay lifetimes • Neutron density 1028/m3 Extremely high flux capture times of the order of fractions of a second Unstable neutron rich nuclei • rapidly decay to form stable neutron rich nuclei • all A<209 and peaks at N=50,82, 126 (magic numbers) 3-38 • • • • • • P process Formation of proton rich nuclei Proton capture process 70<A<200 Photonuclear process, at higher Z (around 40) (g, p), (g,), (g, n) 190Pt and 168Yb from p process Also associated with proton capture process (p,g) Variation on description in the literature 3-39 • Proton-rich nuclei with Z = 7-26 Forms a small number of nuclei with A< 100 • (p,g) and + decays that populate the prich nuclei Also associated with rapid proton capture process • Initiates as a side chain of the CNO cycle 21Na and 19Ne rp process (rapid proton capture) 3-40 Review Notes • Understand Reaction Notation • Understand Energetics of Nuclear Reactions Q values and barriers • Understand the Different Reaction Types and Mechanisms Particles Energy • Relate cross sections to energy • Describe Photonuclear Reactions • Routes and reactions in nucleosynthesis • Influence of reaction rate and particles on nucleosynthesis 3-41 Questions • Describe the different types of nuclear reactions for heavy ions. • Provide notations for the following Reaction of 16O with 208Pb to make stable Au Formation of Pu from Th and a projectile • Find the threshold energy for the reaction of 59Co and an alpha that produces a neutron and a product nuclei • What are the differences between low and high energy reactions? • How does a charged particle reaction change with energy? A neutron reaction? • How are actinides made in nucleosynthesis? • What is the s-process? • What elements were produced in the big bang? • Which isotopes are produced by photonuclear reactions? • What is interesting about the production of 12C 3-42 Question • Respond to PDF Quiz • Comment in blog 3-43