Document
advertisement
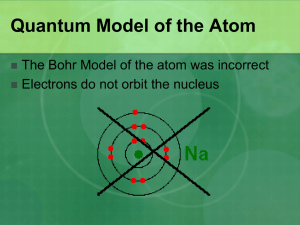
Chapter 7: Quantum Theory and Atomic Structure 7.1 The Nature of Light 7.2 Atomic Spectra 7.3 The Wave-Particle Duality of Matter and Energy 7.4 The Quantum-Mechanical Isaac Newton (1642 – 1727) Max Plank (1858 – 1947) Model of the Atom Erwin Schrödinger (1887-1961) Albert Einstein (1879 –1955) Electromagnetic Radiation: the wave model Christiaan Huygens and Isaac Newton The Wave Nature of Light Visible light is a type of electromagnetic radiation. The wave properties of electromagnetic radiation are described by three variables: - frequency (𝝂), cycles per second - wavelength (λ), the distance a wave travels in one cycle - amplitude, the height of a wave crest or depth of a trough. The speed of light is a constant: c=𝝂xλ = 3.00 x 108 m/s in a vacuum The reciprocal relationship of frequency and wavelength. Differing amplitude (brightness, or intensity) of a wave. Regions of the electromagnetic spectrum Interconverting Wavelength and Frequency Problem 1. Calculate the frequency of each wavelength of electromagnetic radiation: a. 632.8 nm (wavelength of red light from helium–neon laser) b. 503 nm (wavelength of maximum solar radiation) c. 0.052 nm (a wavelength contained in medical X-rays) Problem 2. Calculate the wavelength of each frequency of electromagnetic radiation: a. 100.2 MHz (typical frequency for FM radio broadcasting) b. 1070.0 kHz (typical frequency for AM radio broadcasting) (assume four significant figures) c. 835.6 MHz (common frequency used for cell phone communication) Wavelike properties of light Refraction Dispersion Interference Diffraction Refraction Dispersion Different behaviors of waves and particles: Formation of a diffraction pattern. Diffraction Particle-like properties of light Blackbody radiation Photoelectric effect Compton effect Emission spectra The birth of Quantum Theory (1900) The Quantum Theory of Energy Any object (including atoms) can emit or absorb only certain quantities of energy. Energy is quantized; it occurs in fixed quantities, rather than being continuous. Each fixed quantity of energy is called a quantum. An atom changes its energy state by emitting or absorbing one or more quanta of energy. E = nhn where n can only be a whole number. The Photoelectric Effect (1905) 1. The electrons were emitted immediately - no time lag! 2. Increasing the intensity of the light increased the number of photoelectrons, but not their maximum kinetic energy! 3. Red light will not cause the ejection of electrons, no matter what the intensity! 4. A weak violet light will eject only a few electrons, but their maximum kinetic energies are greater than those for intense light of longer wavelengths! hν = Ek + Φ Problem 3. Calculate the energy of a photon of electromagnetic radiation at the following wavelengths: 632.8 nm (wavelength of red light from helium–neon laser); 503 nm (wavelength of maximum solar radiation); 0.052 nm (a wavelength contained in medical X-rays). Problem 4. A laser pulse with wavelength 532 nm contains 3.85 mJ of energy. How many photons are in the laser pulse? Atomic Spectra Element Abundance (% ) Hydrogen 91.2 Helium 8.7 Oxygen 0.078 Carbon 0.043 Nitrogen 0.0088 Silicon 0.0045 Magnesium 0.0038 Neon 0.0035 Iron 0.030 Sulfur 0.015 Exciting Gas Atoms to Emit Light with Electrical Energy Identifying Elements with Flame Tests Na K Li Ba Three series of spectral lines of atomic hydrogen. Rydberg equation 1 l = R 1 2 n1 1 n22 R is the Rydberg constant = 1.096776x107 m-1 for the visible series, n1 = 2 and n2 = 3, 4, 5, ... Johannes Rydberg (1854 – 1919) The Bohr Model of the Hydrogen Atom (1913) Bohr’s atomic model postulated the following: The H atom has only certain energy levels, which Bohr called stationary states. – Each state is associated with a fixed circular orbit of the electron around the nucleus. – The higher the energy level, the farther the orbit is from the nucleus. – When the H electron is in the first orbit, the atom is in its lowest energy state, called the ground state. The atom does not radiate energy while in one of its stationary states. The atom changes to another stationary state only by absorbing or emitting a photon. – The energy of the photon (hn) equals the difference between the energies of the two energy states. – When the E electron is in any orbit higher than n = 1, the atom is in an excited state. Three series of spectral lines of atomic hydrogen. The visible spectrum A tabletop analogy for the H atom’s energy. 1 l 1 = 1 R n1 2 - n22 1 E = -2.18x10-18 J DE = Efinal – Einitial = -2.18x10-18 J 1 n2final n2 1 n2initial Problem 7. A hydrogen atom absorbs a photon of UV light and its electron enters the n = 4 energy level. Calculate: a) the change in energy of the atom and b) the wavelength (in nm) of the photon. Problem 8. Calculate the wavelength of the light emitted or absorbed when an electron in a hydrogen atom makes each transition and indicate the region of the electromagnetic spectrum (infrared, visible, ultraviolet, etc.) where the light is found. a) n = 2 → n = 1 b) n = 4 → n = 2 c) n = 4 → n = 3 d) n = 5→ n = 6 Emission vs. Absorption Spectra Spectra of Mercury Infrared Spectroscopy The Wave-Particle Duality of Matter and Energy The Wave-Particle Duality of Matter and Energy All matter exhibits properties of both particles and waves. Electrons have wave-like motion and therefore have only certain allowable frequencies and energies. Louis de Broglie l= h mu m = mass u = speed in m/s Problem 9. The smallest atoms can themselves exhibit quantum mechanical behavior. Calculate the de Broglie wavelength (in pm) of a hydrogen atom traveling 475 m/s. Problem 10. A proton in a linear accelerator has a de Broglie wavelength of 122 pm. What is the speed of the proton? Heisenberg’s Uncertainty Principle The paradox solver Heisenberg’s Uncertainty Principle states that it is not possible to know both the position and momentum of a moving particle at the same time. D x∙m D u ≥ h 4p x = position u = speed The more accurately we know the speed, the less accurately we know the position, and vice versa. Problem 11. a) An electron moving near an atomic nucleus has a speed 6x106 m/s ± 1%. What is the uncertainty in its position (Dx)? b) How accurately can an umpire know the position of a baseball (mass = 0.142 kg) moving at 100.0 mi/h ± 1.00% (44.7 m/s ± 1.00%)? The Quantum-Mechanical Model of the Atom Newton’s laws are deterministic Quantum mechanics laws are indeterministic The Quantum-Mechanical Model of the Atom The Schrödinger wave equation allows us to solve for the energy states associated with a particular atomic orbital. The square of the wave function gives the probability density, a measure of the probability of finding an electron of a particular energy in a particular region of the atom. Electron probability density in the ground-state H atom. A radial probability distribution of electrons in hydrogen atom The Schrödinger wave equation solution The radial equation The colatitude equation The azimuthal equation Taken from www.hyperphysics.phy-astr.gsu.edu Where did Quantum Numbers beam from? Taken from www.hyperphysics.phy-astr.gsu.edu 1s, 2s and 3s hydrogen atom orbitals Taken from www.hyperphysics.phy-astr.gsu.edu The 1s, 2s, and 3s orbitals. 2p and 3p hydrogen atom orbitals Taken from www.hyperphysics.phy-astr.gsu.edu The 2p orbitals. 3d hydrogen atom orbitals Taken from www.hyperphysics.phy-astr.gsu.edu The 3d orbitals. The 3d orbitals. The 4fxyz orbital, one of the seven 4f orbitals. Quantum Numbers and Atomic Orbitals An atomic orbital is specified by three quantum numbers. The principal quantum number (n) is a positive integer. The value of n indicates the relative size of the orbital and therefore its relative distance from the nucleus. The angular momentum quantum number (l) is an integer from 0 to (n -1). The value of l indicates the shape of the orbital. The magnetic quantum number (ml) is an integer with 2 x l +1 values, from –l to +l . The value of ml indicates the spatial orientation of the orbital. The angular momentum quantum number (l) is an integer from 0 to (n -1). The value of l indicates the shape of the orbital. L=rxp The magnetic quantum number (ml) is an integer with 2 x l +1 values, from –l to +l . The value of ml indicates the spatial orientation of the orbital. The Hierarchy of Quantum Numbers for Atomic Orbitals Energy shells (levels) and subshells (sublevels) Energetic levels (shells) Energetic sublevel (subshells) Orbitals Main Quantum Number (n) - SHELLS Secondary Quantum Number (l) – SUBSHELLS (l = 0 s subshell; l = 1 p subshell; l = 2 d subshell; l = 3 f subshell) Magnetic Quantum Number (m) – ORBITALS (m = 0 s orbital; m = -1,0,1 p orbitals; m = -2,-1,0,1,2 d orbitals, m = -3,-2,-1,0,1,2 ,3 f orbitals) Problem 12. What values of the angular momentum (l) and magnetic (ml) quantum numbers are allowed for a principal quantum number (n) of 3? How many orbitals are allowed for n = 3? Problem 13. What are the possible values of l for each value of n? a. 1 b. 2 c. 3 d. 4 Problem 14. What are the possible values of m for each value of l? a. 0 b. 1 c. 2 d. 3 Problem 15. Which set of quantum numbers cannot occur together to specify an orbital? In case the set is plausible, give the name of the atomic orbital a. n = 2, l = 1, ml = -1 b. n = 3, l = 2, ml = 0 c. n = 3, l = 3, ml = 2 d. n = 4, l = 3, ml = 0