Great Ideas in Science: Lecture 7 – Chemical
advertisement

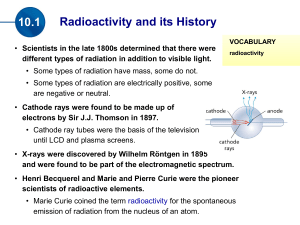
Great Ideas in Science: Lecture 7 – Nuclear Reactions Professor Robert Hazen UNIV 301 Great Idea: Nuclear energy arises from the conversion of mass into energy. Nuclear Reactions Key Idea: Nuclear reactions result from the rearrangement of an atom’s protons and neutrons (i.e. the nucleus) Key Words: • • • • • • • Proton Neutron Nucleus Isotope Radioactivity Nuclear Fission Nuclear Fusion The Building Blocks of Matter Of what is matter made? • Atoms • Nuclei and electrons • Quarks Key Words About Atoms Atom: Any object with a nucleus and electrons Element: An atom with a known number of protons (the atomic number) Ion: An electronically-charged atom with a different number of protons (+) and electrons (-) Isotope: An element with a known number of neutrons The Structure of the Atom Electrons in shells (energy levels) Negatively charged Shift during chemical reactions The Structure of the Atom Electrons in shells (energy levels) Negatively charged Shift during chemical reactions Central dense nucleus Composed of protons and neutrons Positively charged Nucleus - Stays put in chemical reactions Isotopes: Hydrogen & Carbon H-1 – 1 proton H-2 – 1 p & 1 neutron (Deuterium) H-3 – 1 p & 2 n (Tritium) C-12 – 6p & 6n C-13 – 6p & 7n C-14 – 6p & 8n (radioactive) For any given element the number of protons is fixed Four Fates of Isotopes An isotope may be stable An isotope may be radioactive An isotope may be split apart by fission An isotope may combine with another by fusion Chart of the Isotopes (Z vs. N) Stable Isotopes 99.999+% of all the atoms around us Examples are carbon-12 and carbon-13 Different isotopes don’t affect chemical reactions. Used in scientific research to track chemical reactions (2 ways) • As tracers • Fractionation Radioactivity or Radioactive Decay (three kinds) The spontaneous emission of an energetic particle by a nucleus Alpha radiation Beta radiation Gamma radiation Most Kinds of Isotopes are Radioactive STABLE RADIOACTIVE Alpha Radiation Atom spontaneously loses 2 protons and 2 neutrons (= a Helium-4 nucleus) Alpha Radiation Atom spontaneously loses 2 protons and 2 neutrons (= a Helium-4 nucleus) Uranium-238 Thorium-234 + 2n + 2p Beta Radiation One neutron spontaneously becomes a proton plus an electron Thorium-234 Proactinium-234 Gamma Radiation Atom spontaneously emits a gamma ray (electromagnetic radiation) Uranium-238* Uranium-238 + γ Gamma Radiation Atom spontaneously emits a gamma ray (electromagnetic radiation) Uranium-238* Thorium 234 + γ SUMMARY: The Three Kinds of Radioactive Decay Alpha Decay • Release of α particle with 2 protons and 2 neutrons Beta Decay • Neutron becomes a proton • Emission of electron (β-ray) Gamma Radiation • Electromagnetic radiation Radioactivity and Health Ionization • Stripping off electrons Long-term effects • Cancer • Birth defects Half-Life The average time for decay of ½ batch of radioactive isotopes Wide range of half-lives Radiometric Dating 1. Know half-life of isotope 2. Know how much was there 3. Measure what’s left Carbon-14: Half-life = 5730 years Radiometric Dating Applications to geology • Need longer half-lives • Uranium, potassium Radioactive Decay Chain (radon) Radioactive Decay Chain (radon) Radioactive Decay Chain (radon) Radioactive Decay Chain (radon) Radioactive Decay Chain (radon) Radioactive Decay Chain (radon) Radioactive Decay Chain (radon) Radioactive Decay Chain (radon) Radioactive Decay Chain (radon) Radioactive Decay Chain (radon) Radioactive Decay Chain (radon) Radioactive Decay Chain (radon) Radioactive Decay Chain (radon) Four Fates of Isotopes An isotope may be stable An isotope may be radioactive An isotope may be split apart by fission An isotope may combine with another by fusion Nuclear Fission (Splitting) Fission = Splitting of nucleus A nuclear reactor converts mass to energy Nuclear Fission (Splitting) Nuclear Fission – The Atom Bomb Hiroshima – August 6, 1945 Nuclear Fission – The Atom Bomb Yucca Mountain, Nevada (NIMBY) Yucca Mountain, Nevada (NIMBY) Four Fates of Isotopes An isotope may be stable An isotope may be radioactive An isotope may be split apart by fission An isotope may combine with another by fusion Nuclear Fusion (Fusing) • Hydrogen atoms combine to form helium • Some mass is converted into energy Nuclear Fusion – Hydrogen Bomb Nuclear Fusion – Hydrogen Bomb Stars are Giant Fusion Reactors http://www.earth.northwestern.edu/people/seth/107/Solar/Image12.gif Fates of Stars Benefits of Isotopes Stable Isotopes • Medical Research • Environmental Tracers Radioactive Isotopes • • • • Medical diagnosis Cancer treatments Environmental tracers Age Determination Nuclear fission • Power generation Nuclear Fusion • The Sun