Super-Resolution Fluorescence Microscopy
advertisement

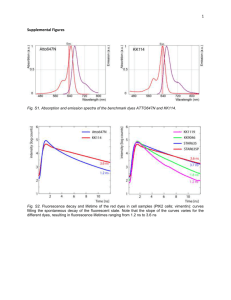
Super-Resolution Fluorescence Microscopy Clif Thivierge 08/12/10 Prof. Kevin Burgess Texas A&M University The Bane of Imaging: Diffraction Limit practical limit obtained when imaging very small objects by magnification diffraction causes blurring of objects when imaging smaller than ~200-500 nm (diffraction limit) “broadening” of a point caused by diffraction is known as the “point spread function” () x-y = (0.61 )/( sin()) = refractive index medium = half-cone angle of focused light Examples of Diffraction Limit Ways to Circumvent Limit • Near Field Microscopy (NSOM) • Far Field Microscopy – Confocal, 4pi and I5M, SIM • Super-Resolution – Spatially Patterned Excitation • STED • RESOLFT • SSIM – Localization Methods • STORM • PALM • FPALM Near Field Imaging (NSOM) -place microscope distance less than 1 wavelength from sample -20-50 nm resolution QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. problem: cannot image into sample because of wavelength restriction Far Field: Confocal Microscopy • Non-linear 2-photon excitation and pinhole detection decrease SPF beyond classical limits • 21/2 improvement in resolution QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. problem: 2-photon excitation uses high wavelengths which increase SPF: x-y = (0.61 )/( sin()) Structured-Illumination Micropscopy (SIM) 100 nm resolution possible QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Conclusions • methods use common dyes (good) • confocal is easiest, most widely used • best resolution obtainable only 100 nm (SIM) • single molecule is problematic Super-Resolution Microscopy Goal: obtain sub-100 nm resolution pioneered by Stefan Hell in mid-1990s Max Plank Institute (Germany) two methods: (i) Spatially Patterned Excitation STED, RESOLFT, SSIM (ii) Localization Methods STORM, PALM, FPALM QuickTime™ and a T IFF (Uncompressed) decompr essor ar e needed to see t his pictur e. Stimulated Emission Depletion Microscopy (STED) Spontaneous VS Stimulated Emission QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. when an excited molecule encounters a photon matching it’s emission energy, another “clone” photon is created and ground state results STED (i) sample is excited with laser and blur is obtained due to diffraction (exc) (ii) another “doughnut” shaped laser excites at emission wavelength (STED) of dye and switches outside dyes to “dark state” (iii) observing in between exc and STED, very resoved image is produced observe QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. exc STED STED Microscopy resolutions 20 - 30 nm common, best 6 nm STED Dyes -dyes need to be very photostable excitation laser: 107 W/cm2 STED laser: 109 W/cm2 -dye needs large stimulated emission cross-section -most common: Atto 532 and Atto 647N Rhodamine derivatives? Dyes Used for STED QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Reversible Saturable Optically Linear Fluorescence Transitions (RESOLFTs) same concept as STED but dyes are made to “dark state” by other mechanisms: -switch to triplet state -switch to ground state -use reversibly photoswitchable dyes advantages: -less powerful lasers need to be used (100 W/cm2) -this leads to many more dyes and even fluorescent proteins being used Single Molecule Imaging the exact location of single dyes can be determined by doing multiple excitation/emission cycles Things Become More Complicated with Multiple Dyes Considerable overlap. Hard to identify individual fluors in real live. Super-Resolution Single Molecule Localization Methods • Stochastic Optical Reconstruction Microscopy (STORM) • Photoactivated Localization Microscopy (PALM) • Fluorescence Photoactivated Localization Microscopy (FPALM) all work by same principle: image only some dyes at one time Consider Previous Example Considerable overlap. Hard to identify individual fluors in real live. Most Dyes in “Off-state” localization of “on-state” dyes possible Switch the State of the Dyes localization of other dyes possible Combine the Images position of each dye is known Dyes for Localization Microscopy • have on and off state • easily able to switch from on/off state • on/off can be non-fluorescent or have a change in either excitation or emission wavelengths • best if reversible but not necessary Common Dyes for Localization Mic. Examples of Photoswitchable Dyes Conclusions on Localization Microscopy QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. using this technique 3D localization of labels can be achieved with 20 nm resolution Multicolor Imaging methods discussed so far are valuable in the elucidation of structures to study interactions, multicolor imaging can be used multicolor imaging has been done with both STED and Localization Microscopy Multicolor STED 2 methods: (i) use set of dyes with non-overlapping excitation, emission, and STED wavelengths (hard to find) (ii) find dyes with same STED excitation but non-overlapping absorbance Multicolor STED: Synaptic Proteins Synaptophysin (red) Syntaxin 1 (green) Conclusions still very young technology and best dyes have yet to be discovered impact is big since imaging is such a popular tool a lot of opportunity for innovation/development The End