File
advertisement

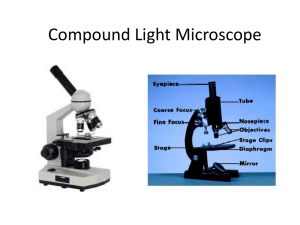
CHAPTER 18 Techniques in Cell and Molecular Biology Introduction • Research in cell biology requires complex instrumentation and techniques. • Understanding the technology helps in understanding the cell. 18.1 The Light Microscope (1) • The light microscope uses the refraction of light rays to magnify an object. – A condenser directs light toward the specimen. – The objective lens collects light from the specimen. – The ocular lens forms an enlarged, virtual image. The paths taken by light rays to form an image The Light Microscope (2) • Resolution – Resolution is the ability to see two nearby points as distinct images. • The numerical aperture is a measure of the lightgathering qualities of a lens. • The limit of resolution depends on the wavelength of light. • Optical flaws, or aberrations, affect resolving power. Resolution The Light Microscope (3) • Visibility – Visibility deals with factors that allow an object to be observed. • It requires that the specimen and the background have different refractive indexes. • Translucent specimens are stained with dyes. • A bright-field microscope a light that illuminates the specimen is seen as a bright background; it is suited for specimens of high contrast such as stained sections of tissues. The Feulgen stain The Light Microscope (4) • Preparation of Specimens for Bright-Field Light Microscopy – A whole mount is an intact object, either living of dead. – A section is a very thin slice of an object. • To prepare a section, cells are immersed in a chemical called a fixative. • The rest of the procedures minimize alteration from the living state. The Light Microscope (5) • Phase-Contrast Microscopy – The phase-contrast microscope makes highly transparent objects more visible by converting differences in the refractive index of some parts of the specimen into differences in light intensity. – Differential interference contrast (DIC) optics gives a three-dimensional quality to the image. A comparison of cells seen with different types of light microscopes The Light Microscope (6) • Fluorescence Microscopy (and Related Fluorescence-Based Techniques) – Fluorescence microscopy has made possible advances in live-cell imaging. – Fluorochromes are compounds that release visible light upon absorption of UV rays. – Fluorochrome stains cause cell components to glow, a phenomenon called fluorescence. The Light Microscope (7) • Fluorescence microscopy (continued) – Fluorochrome-conjugated antibodies are used to locate specific cellular structures (immunofluorescence). – The gene for green fluorescent protein (GFP) from jellyfish can be recombined with genes of interest in model organisms. • GFP is expressed with the host gene of interest. • GFP is used to follow a gene of interest. Use of GFP variants to follow the dynamic interactions between neurons and target cells in vivo The Light Microscope (8) • Fluorescence microscopy (continued) – A GFP variant is called fluorescence resonance energy transfer (FRET), which uses fluorochromes to measure changes in distance between labeled cellular components. The Light Microscope (9) • Video Microscopy and Image Processing – Video microscopy is used to observe living cells. – Video cameras offer several advantages for viewing specimens. • They can detect and amplify very small differences in contrast. • Images produced by video cameras can be converted to digital electronic images and processed by a computer. The Light Microscope (10) • Laser Scanning Confocal Microscopy – A laser scanning confocal microscope produces an image of a thin plane located within a much thicker specimen. – A laser beam is used to examine planes at different depths in a specimen. Laser scanning confocal fluorescence microscopy The Light Microscope (11) • Super-Resolution Fluorescence Microscopy – STORM (stochastic optical reconstruction microscopy) allows the localization of a single fluorescent molecule within a resolution of <20 nm. – Fluorescent images can be positioned with greater accuracy. Breaking the light microscope limit of resolution 18.2 Transmission Electron Microscope (1) • Transmission electron microscopes (TEMs) use electrons instead of light to form images. – The limit of resolution is about 10-15 Å. Transmission Electron Microscope (2) • The components of an electron microscope: – An electron beam from a tungsten filament accelerated by high voltage, and focused with a magnetic field. – A condenser lens is placed between the electron source and the specimen. – Differential scattering of electrons by the specimen creates the image. • Proportional to the thickness of the specimen. • Tissues are stained with heavy metals for contrast. A comparison of the lens system of a light and electron microscope Transmission Electron Microscope (3) • Specimen Preparation for Electron Microscopy – Specimens must be fixed, embedded, and sectioned thinly. • Glutaraldehyde and osmium tetroxide are common fixatives. • Specimens are dehydrated prior to embedding. • Epon or Araldite are common embedding resins. • Thin sections cut with glass or diamond knives are collected on grids. Preparation of a specimen for observation in the electron microscope Transmission Electron Microscope (4) • Specimen preparation (continued) – Chemicals used may cause an artifact, which may be disproved by using other techniques. – In negative staining, heavy metal diffuses into spaces between specimen molecules. – Shadow casting coats a specimen with metal to produce a three-dimensional effect. Examples of negatively stained and metal-shadowed specimens The procedure used for shadow casting Transmission Electron Microscope (5) • Freeze-Fracture Replication and FreezeEtching – In freeze-fracture replication, frozen tissue is fractured with a knife. • A heavy-metal layer is deposited on fractured surface. • A cast of the surface is formed with carbon. • The metal-carbon replica is viewed in the TEM. – In freeze-etching, a layer of ice is evaporated from the surface of the specimen prior to coating it with heavy metal. Procedure for the formation of freezefracture replicas Freeze-fracture and freeze-etching 18.3 Scanning Electron Atomic Force Microscopy (1) • Scanning electron microscopes (SEMs) form images from electrons that have bounced off the surface of a specimen. – Specimens for SEM are dehydrated by criticalpoint drying. – Specimens are coated with a layer of carbon, then gold. – The image in SEM is indirect. – SEM has a wide range of magnification and focus. Scanning electron microscopy Scanning Electron Atomic Force Microscopy (2) • Atomic Force Microscopy – The atomic force microscope (AFM) is a highresolution scanning instrument. – AFM provides an image of each individual molecule as it is oriented in the field. 18.4 The Use of Radioisotopes (1) • Radioisotopes can be easily detected and quantified. – Properties of radioisotopes: • An isotope refers to atoms that differ in the number of neutrons. • Isotopes with an unstable combination of protons and neutrons are radioactive. • The half-life of a radioisotope measures its instability; half of the radioactive material disintegrates in a given amount of time. Properties of a variety of radioisotopes The Use of Radioisotopes (2) • Liquid scintillation spectrometry – Scintillants absorb the energy of an emitted particle and release it in the form of light. – Radiation of a tracer in a sample can be detected by measuring light emitted by a scintillant. The Use of Radioisotopes (3) • Autoradiography is a technique to where a particular isotope is located. – A particle emitted from a radioactive atom activates a photographic emulsion. – The location of the radioisotope in the specimen is determined by the positions of the overlying silver grains in a photographic emulsion. Preparation of light microscopic autoradiograph Examples of autoradioagraphs 18.5 Cell Culture (1) • Most of the study of cells is carried out using cell culture. – Cells can be obtained in large quantities. – Most culture contain a single type of cell. – Many different types of cells can be grown in culture. – Cell differentiation can be studied in a cell culture. • Cells in a culture require media that includes hormones and growth factors. Cell Culture (2) • A primary culture is when cells are obtained directly from the organism. • A secondary culture is derived from a previous culture. • A cell line refers to cells with genetic modifications that allow them to grow indefinitely. • Many types of plant cells can be grown in culture. Cell Culture (3) • A two-dimensional culture system is when cells are grown on the flat surface of a dish. • Labs are moving to three-dimensional cultures in which cells are grown in a 3D matrix consisting of extracellular materials. • 3D cultures are better suited to study cell-cell interactions. A comparison of cell morphology of cells growing in 2D versus 3D cultures 18.6 The Fractionation of a Cell’s Contents by Differential Centrifugation • Differential centrifugation facilitates the isolation of particular organelles in bulk quantity. – Prior to centrifugation, cells are broken by mechanic disruption in a buffer solution. – The homogenate is subjected to a series of sequential centrifugations. – Organelles isolated can be used in a cell-free system to study cellular activities