HERE - Physics Department
advertisement

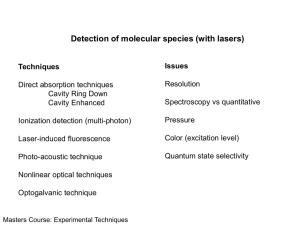
Laser Spectroscopy Group Department of Physics NUI - University College Cork Cork, Ireland Research Activities • Molecular Absorption Spectroscopy • Luminescence Excitation Spectroscopy • Third-order Nonlinear Susceptibilities (Zscan) • Synthesis of Metal Nanoparticles by Laser Ablation • Surface Enhanced (Resonance) Raman Spectroscopy Molecular Absorption Spectroscopy • Cavity Ring-Down Spectroscopy (!) Dye laser based (vis): Static gas, supersonic jets. • Cavity Enhanced Absorption Spectroscopy Diode laser based (near IR): Static gas, flow chamber. • Incoherent Broad-Band Cavity Enhanced Absorption Spectroscopy Xenon Lamp based (UV / vis / near IR): Static gas. [S.E. Fiedler, A. Hese, A.A. Ruth; Chem. Phys. Lett. 371 (2003) 284-294.] Cavity Ring-Down Spectroscopy Cavity-Ring Down (CRD): Highly sensitive direct absorption method for species in the gas-phase. In CRD spectroscopy the rate rather than the absolute magnitude of a change of intensity is determined. Advantages: • intensity independent (in principle) • very long path-lengths • high spectral resolution possible • applicable over a wide spectral range Principle of Cavity Ring-Down (CRD) laser pulse of a dye laser mirror (R>0.999) rel. intensity 1,0 I0 I1 I2 ….. In 1 I0 0,8 1 I2 0,6 0,4 In 0,2 0,0 0 no absorption I1 40 60 time / s 80 100 I = A exp(-t/crd) crd / s -1 fit 20 -1 / nm 1 crd (1 R) c ( ) c Principle of Cavity Ring-Down (CRD) laser pulse of a dye laser mirror (R>0.999) rel. intensity 1,0 I0 I1 I2 ….. In 2 I0 0,8 2 I2 0,6 0,4 0,2 0,0 0 absorption I1 40 60 time / s 80 100 I = A exp(-t/crd) crd / s -1 fit 20 -1 / nm 1 crd (1 R) c ( ) c Examples of investigations using Cavity Ring-Down Spectroscopy • Spin forbidden transitions in aromatic thiocarbonyl S S compounds (in jet and static cell) O O A.A. Ruth, W.G. Doherty, R.P. Brint; Chem. Phys. Lett. 352 (2002) 191-201. A.A. Ruth, T. Fernholz, R.P. Brint, M.W.D. Mansfield; J. Mol. Spectr. 214 (2002) 80-86. • Fast decay dynamics in jet-cooled azulene A.A. Ruth, E.-K. Kim, A. Hese; Phys. Chem. Chem. Phys. 22 (1999) 5121-5129. • Nonlinear dynamics of UV multiphoton photolysis products of gaseous naphthalene A.A. Ruth, E.W. Gash, M. Staak, S.E. Fiedler; Phys. Chem. Chem. Phys. 4 (2002) 5217-5220. Experimental Setup Dye Laser Excimer Laser (XeCl, 308 nm) Probe HR Mirror Iris Shutter Pump Vacuum Cell Lens Filter PMT Computer GPIB Digital Oscilloscope Excimer Laser Shutter / Lens Dye Laser PMT CRD Mirror CRD Mirror Oscillations 8 793 s Conditions: 561 s 6 5 Pnap PHe EPuls T 494 s 483 s 4 = 0.10 mbar = 77.5 mbar = 21.4 mJ = 24.1 oC -7 10 / cm -1 7 3 2 1 0 0 20 UV Pulses 1 2 3 time / hrs. 4 5 Investigations using Cavity Enhanced Absorption Spectroscopy Investigations using Cavity Enhanced Absorption Spectroscopy High spectral resolution (~60 MHz) rel. absorbance • Water vapour overtones in ambient air (vis) • Formaldehyde (~1.5 m) at 4 mbar 1511.4 nm 20 1510.8 nm rotationally resolved 15 10 5 6616.5 6617.0 6617.5 -1 ~ / cm 6618.0 6618.5 Z-scans of Pt-octaethylporphyrin in toluene Open aperture: | | = (3.97±0.09) x 10-9 m W-1 Closed aperture: ’= 7.7 x 10-16 m2 W-1 1.3 norm. transmission norm. transmission 1.4 1.3 1.2 1.1 1.2 1.1 1.0 0.9 1.0 0 10 20 30 40 50 z / mm Pt-OEP in toluene (8.6 x 10-5 M) 0.8 0.0 0.5 1.0 1.5 2.0 2.5 z / mm Energy per pulse 8 J. 3.0 Synthesis of metal nanoparticles by laser ablation irises ····· ········ · Nd:YAG ···· · · ····· · 532 nm metal (target) x cell H2 O y z optical density lens 1.4 1.2 1.0 0.8 0.6 0.4 0.2 0.0 200 400 600 / nm 800 <d>~30 nm Group Members Academic Staff Prof. M. Mansfield (general supervision) Post-Doctoral Fellows To be appointed ( CEAS and IBBCEAS – multi-component trace gas detection) PhD Students E. Gash (CRD, nonlinear dynamics of naphthalene) M. Staak (CEA, formaldehyde, photolysis products - HO2) K. Lynch (Surface enhanced Raman scattering) One position vacant Masters Students S. O’Brien (z-scan measurements of porphyrin solutions) A. Walsh (z-scan measurements of porphyrin thin films) [ R. Healy (Nanoparticle formation by laser ablation – alloys) ] Research Collaborations University College Cork – Physics Department Prof. D. Nikogosyan, Dr. A. Dragomir (nonlinear absorption measurements) University College Cork – Chemistry Department Prof. P. Brint (LIF, LIP, forbidden transitions, supersonic jets) Prof. J. Sodeau, Dr. J. Wenger (atmospheric chemistry) Dr. J. Holmes, Dr. M. Morris, Prof. T. Spalding (nanoparticles and nanostructures, TEM) Prof. D. Burke (laser assisted electrocatalysis) Technical University Berlin, Germany Prof. A. Hese, S. Fiedler (CRD spectroscopy, IBBCEAS) Max-Planck-Inst. Biophys. Chemistry, Göttingen, Germany Prof. J. Troe (liquid phase spectroscopy and kinetics) Univ. Paris-Sud (Dr. Orphal), Niels-Bohr-Inst. Copenhagen (Prof. Heimburg), Warsaw University (Dr. Borowicz) …. etc. Future activities Multi-component gas analysis using Incoherent BroadBand Cavity Enhanced Absorption Spectroscopy Detection of NO2, NO3, O3 , H2O, HONO … (vis) Alloy nanoparticle synthesis New materials for SERS Surface Enhanced (Resonance) Raman Scattering Porphyrins and proteins on particle surfaces