DE = hn em E 2
advertisement

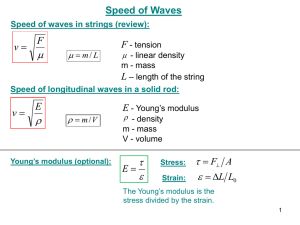
Spectroscopic Analysis Part 3 – Spectroscopy Experiments Chulalongkorn University, Bangkok, Thailand January 2012 Dr Ron Beckett Water Studies Centre School of Chemistry Monash University, Melbourne, Australia Email: Ron.Beckett@monash.edu Water Studies Centre 1 Energy is Quantized The energy of atoms and molecules is quantized. They can only exist in allowed energy states or levels Electronic energy levels in a H atom 1s 2s 2p 3s 3p The lowest energy state has the single electron in the 1s orbital 1s1 2 Absorption and Emission of EMR When EMR is absorbed or emitted by matter is does so in whole photons only (NOT fractions) Absorption involves promotion from a lower energy state to a higher one Emission results in a jump from a higher energy level to a lower energy level E2 DE = hn E1 E2 DE = hn E1 3 E2 DE = hn Intensity E1 n Frequency Intensity E2 DE = hn n E1 Frequency 4 1. Absorption Spectroscopy Experiments Slit Monochromator Detector Light Source Sample Slit Recorder 5 Techniques of Wavelength Selection 1. Filters • Absorption filters – Coloured glass or gelatin – Normally broad spectral bandwidth • Cutoff or bandwidth filters – Can be combined to provide narrower bandwidth 6 Techniques of Wavelength Selection 2. Prism Monochromators 2 Entrance slit Collimating lens 1 Prism Bunsen prism monochromator Focussing lens Focal plane Slit 7 Techniques of Wavelength Selection 3. Diffraction Grating Monochromators – Glass or plastic plate covered with fine lines – Reflect light of different wavelengths at different angles. Condition for constructive interference (transmission) must be achieved where the path difference between adjacent beams must be an integral number of wavelengths n = d(sin i + sin r) where n is the diffraction order . n=1 i n=2 r 8 3. Diffraction Grating Monochromators Concave mirrors Reflection Grating Entrance slit 1 Exit slit 2 9 3. Diffraction Grating Monochromators 10 EMR Detectors for Spectroscopy 1. Photographic Plates 11 EMR Detectors for Spectroscopy 2. Phototubes and Photomultipliers Use the photoelectric effect to convert photons into a measureable electric current 12 EMR Detectors for Spectroscopy 3. Silicon Photodiodes Consist of a p-n silicon junction which increases in conductivity when exposed to UV-visible radiation. The change in conductivity is used to measure the light intensity. Photo Diode Array Detectors A series of such photodiodes can be constructed and used to simultaneously detect the radiation of different wavelengths separated by a 13 monochromator Origin of an Absorption Peak Energy Transition E2 DE = hn E1 Absorption Spectrum Intensity n Frequency 14 Absorption Spectrum 15 Absorption Spectrum 16 2. Emission Spectroscopy Experiments Sample Monochromator Detector Excitation Energy Heat Electrical EMR Slit Recorder 17 Origin of an Emission Peak Energy Transition E2 DE = hn Excitation E1 Emission Spectrum Intensity n Frequency 18 3. Fluorescence Spectroscopy Experiments Monochromator Sample nem Detector nex Slit Slit Monochromator Slit Recorder Light Source 19 Origin of a Fluorescence Peak E2 Energy Transition E3 DE = hnex Radiationless energy loss DE = hnem E1 Emission Spectrum Intensity nem Frequency 20 21 Forensic Application of Fluorescence Visualization of fingerprints Fingerprint visualized by redwop fluorescent fingerprint powder Cyanoacrylate fumed + Rhodamine 6G Cyanoacrylate fumed 22 4. Chemiluminescence Excitation to a higher molecular electronic state by a chemical reaction followed by emission of EMR E2 Excitation by a chemical reaction D E = hn E1 23 Chemiluminescence Observed in Nature e.g. firefly, fungi, jellyfish, bacteria, crustacea and fish all may exhibit bioluminescence. 24