Growing old is bad enough, who ordered senility?
advertisement

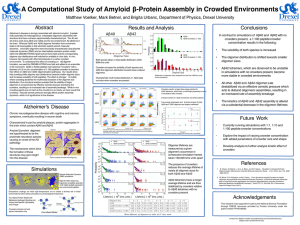
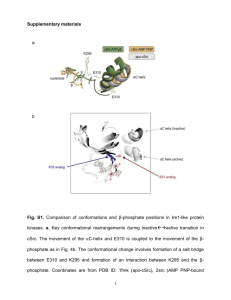
Not just Cheaper. Better. Sudipta Maiti Tata Institute of Fundamental Research, Mumbai 30 Years of ASET, TIFR, 18Feb13 Biology is not about cutting frogs anymore: Actually, it never was Robert Hooke’s microscope Source: Wikipedia There is a revolution on in microscopy Label-free Multi-Photon microscopy of serotonin Sarkar et al. Frontiers in Membrane Physiology (2012) Measuring sub-nm size, inside water “Fluorescence Correlation spectrometer (FCS)” Magde et al. (1972) Sengupta et al., Methods (2002) Cool Tools S. Hell (2009) You are only as good as your microscope Combining FCS and Multiphoton An alignment-free instrument with high sensitivity Kaushalya et al., US Patent no. 7,705,987 (2010) FCS Workshop 2009 Teaching colleagues from 10 institutes how to build their own Sensitivity > commercially available Cost < 1/8 th Why couldn’t they do it before? A culture of building instruments Source: JPK website) Cutting edge technology is only available in specific labs – until it is marketed When are our best labs going to put India on the map of cutting edge scientific instruments? Perhaps soon! i2n Technologies, Bangalore Holmarc, Kochi (Technology from TIFR) Not just cheaper. Better. A dual objective set-up: Half a million photons/sec from a single molecule (fast) Auto aligned 4 collection (Picosecond) Abhyankar et al. , Proc. SPIE (2012) Why should we do it? • A step forward for someone else to build up the knowledge base • Recognition from peers all over the world • Promote the culture of instrument building among Indian labs and companies • Contribution to the economy (?) Who should do it? With its extra-ordinary legacy of developing scientific instruments, TIFR MUST TAKE A LEAD Thanks to all my students and collaborators Thank you Folding intermediates: progress in silico Folding of villin headpiece Computational Biophysics Group, UIUC Experimentalists are far from verifying it Optical: Fast, low resolution, NMR: Slow, high resolution Fragments: concentration can change folding rate X Separation Unfolding Normal Amyloid aggregation aggregation Self-complimentarity Wolynes and coworkers, PNAS (2013) Amyloids: Aggregation and folding are intertwined Concentration affects Amyloid-β aggregation kinetics Oligomers 150nM Monomer 15 nM Nag et al., J. Biol. Chem. (2011) Why are we interested in Aβ oligomers? Amyloid-β intermediates are VERY interesting Bio-activity <100 nm Coles et al. Biochemistry (1998) Crescenzi et al. Eur. J. Biochem (2002) <10 nm Petkova et al. PNAS (2002) Aggregation Number (FCS) How do we measure things at a sub-resolution level? Time ( min) Photon bunching Diffusion time Time ( µs) Emitted photons Avg. fluorescence Emitted photons Emitted photons Photon statistics: Local excitation in a fluorescent solution Anti-bunching lifetime Time ( ns) Auto-Correlation: extracting timescales of processes Fluorescence photon bunching and anti-bunching Lifetime (Confor mation) Diffusion (Size) Abhyankar et al. , Proc. SPIE (2012) Folding: FRET measures conformation change Monomer Oligomer The monomer is “open”, while the oligomer (tetramer or larger) is a “closed” structure The major conformational change is between the monomer and the small oligomer, it remains similar thereafter 20 Need more detailed, more robust information 300K, FCS measures size as a function of time 78K, flash-frozen at appropriate size 240K, lyophilized ssNMR (with P. K. Madhu) The ssNMR-derived oligomer structure PDB : 2 BEG, Riek and Coworkers Tertiary F19-L34 contact is also present Structure similar to fibrils found earlier Mithu et al., Biophys. J., 2011 ssNMR shows that the small oligomer has a conformation broadly similar to the fibril Untreated 150 nM Abeta treated Scale Bar ~ 10 µm A mixture of Aβ monomers and oligomers can bind to cell membranes Nag et al., Biophys. J. (2010) 23 But everyone has the monomers?! Do Aβ monomers bind to membranes? Monomers , HEK cells 0 minute 30 minute Oligomers (same concentration as monomers) 24 Membrane affinity drastically increases as monomers become oligomers 3) Which part of the molecule is the key? Looking at the core only : the short “S” peptide M L G I AβS – 18-35 residues I A G K N S V F F A E D V G Folds into a hairpin very similar to the full length Aβ Muralidharan et al., Chem. Phys. (2013), in press A40 0 Minutes 30 Minutes AS Control » But toxicity requires the unstructured part… Percent Cell viability 100 90 80 70 60 50 40 30 20 10 0 CTL Aβ40 Aβ10-40 Aβ14-40 Aβ17-40 Aβ22-40 S Membrane binding may be necessary, but it is not sufficient for toxicity N-terminal part is required for subsequent events A dominant model for toxicity is the leakage of neurotransmitters from vesicles Also, analysis shows neurotransmitter packaging-related genes are affected » The Questions and the answers 1) At what stage of aggregation does the molecule fold? As early as tetramer , perhaps earlier 2) Is there an intermediate structure? None detected 3) Does folding determine bioactivity? Yes, it seems to be required for membrane attachment 4) Which part of the molecule is the key? The core (18-35) determines folding and membrane attachment, but unstructured N-terminus required for toxicity The human parts which made this possible: Acknowledgements: Venus Singh Mithu P. K. Madhu C. Muralidharan S. Dandekar V. Vaidya D. Khushalani G. Walker Elisha Haas Eitan Lerner G. Krishnamoorthy M. Kombrabail (left to right) Christina McLaughlin, Bidyut sarkar, Debanjan Bhowmik, Anand Kant Das, SM, C. Muralidharan, Bappaditya Chandra Also, Rajiv Abhyankar, and Suman Nag (Now in Stanford) Lalit Borde National NMR Facility Funding: DIT, DBT, TIFR 29 TIRF measures ms vesicle docking events at the membrane Experiments with Amyloids are going on…. Even artificial SUVs show the same effect A rapid, cell free assay for Aβ bioactivity 31 Challenge: Excitation is in UV, but UV kills Solution: Multiphoton excitation (here 3-photon excitation with 740nm) Serotonin 350 nm 1.0 ES Normalised Units 0.8 0.6 270 nm hν/3 hν 0.4 hν2 Intensity high enough to cause UV excitation 0.2 GS 0.0 240 300 360 420 Wavelength (nm) 480 Maiti et al., Science , 1997 Kaushalya et al., J. Neurosci. Res. (2008) Localized Excitation The ssNMR-derived oligomer structure PDB : 2 BEG, Riek and Coworkers Tertiary F19-L34 contact is also present Structure similar to fibrils found earlier Mithu et al., Biophys. J., 2011 ssNMR shows that the small oligomer has a conformation broadly similar to the fibril » The Questions: 1) Does oligomer formation involve folding? 2) Is this structural change linked to function? 3) Which part of the peptide is responsible for which property? The Solutions: 1) 2) 3) 4) Size by FCS (Fluorescence Correlation Spectroscopy) Conformation by FRET (Forster Resonance Energy Transfer) Detailed conformation by solid state NMR (Flash-freezing after 1&2) Bio-activity by confocal (membrane attachment) and multiphoton microscopy (neurotransmitter imaging) How do you do it experimentally ? A single molecule level “Fluorescence Correlation spectrometer” Magde, Elson and Webb, PRL (1972) Review: Maiti, Haupts and Webb, PNAS (1997) Combined FCS, Antibunching and TCSPC (lifetime): Simultaneously measuring size and conformation (fast) Auto aligned 4 collection (Picosecond) Abhyankar et al. , Proc. SPIE (2012) Time ( min) Photon bunching Diffusion time Time ( µs) Emitted photons Avg. fluorescence Emitted photons Emitted photons Photon statistics: Local excitation in a fluorescent solution Anti-bunching lifetime Time ( ns) Conformation: Are the oligomers differently folded? Forster Resonance Energy Transfer (FRET) Acceptor DONOR Lifetime measures energy transfer End-to-end distance |S1> Dipole-dipole energy transfer efficiency ~ 1/ R6 kTr Excitation kR kNR |S0> 38 A nanometric ruler for interchromophoric distance FÖrster (1948); Haugland and Stryer (1976) misfolding The process preserves the oligomers Before Lyophilization After Lyophilization