Section 10.3
advertisement

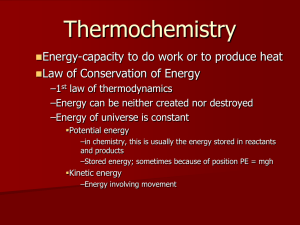
Friday, Oct. 25th: “A” Day Monday, Oct. 28th: “B” Day Agenda Homework questions/Quick review Section 10.2 Quiz: “Using Enthalpy” Section 10.3: “Changes in Enthalpy During Chemical Reactions” Calorimetry, calorimeter, adiabatic calorimetry, Hess’s Law, standard enthalpy of formation Homework Pg. 15 practice worksheet (MUST show work) Sec. 10.3 review, pg. 357: #1-5 Concept Review: “Changes in Enthalpy During Chemical Reactions” Homework Questions/Problems Pg. 349: #1-8 Section 10.2 Quiz: “Using Enthalpy” You can use both your book and your notes. You’ll need both 10.1 AND 10.2 notes. May the FORCE be with you! #4: M = molar mass #8: Use the “25 J rule” Changes in Enthalpy Accompany Reactions Changes in enthalpy occur during chemical reactions. A change in enthalpy during a reaction depends on many variables, but temperature is one of the most important variables. To standardize enthalpies of reaction, data are presented for reactions in which both reactants and products have the standard thermodynamic temperature of 25˚C or 298.15 K. Chemical Calorimetry Calorimetry: the measurement of heatrelated constants, such as specific heat or latent heat. Calorimeter: a device used to measure the heat absorbed or released in a chemical or physical change. Nutritionists Use Bomb Calorimetry A bomb calorimeter is used to measure enthalpy changes caused by combustion reactions. Adiabatic Calorimetry is Another Strategy Instead of using a water bath to absorb the energy generated in a combustion reaction, adiabatic calorimetry uses an insulating vessel that doesn’t allow energy to pass through. As a result, the temperature of the reaction mixture will change and can be recorded. Adiabatic calorimetry is used for reactions that are not ignited, such as for reactions in aqueous solution. Hess’s Law Hess’s Law: the law that states that the amount of heat released or absorbed in a chemical reaction does not depend on the number of steps in the reaction. The overall enthalpy change in a reaction is equal to the sum of the enthalpy changes for the individual steps in the process. Standard Enthalpies of Formation Standard enthalpy of formation: the enthalpy change in forming 1 mol of a substance from elements in their standard state. By definition, the values of the standard enthalpies of formation for elements are zero. Symbol: ΔH˚f Unit: kJ/mol Calculating Enthalpy Change for a Chemical Reaction Using a list of standard enthalpies of formation, the enthalpy change of any reaction for which there is data available can be calculated: ΔHreaction = ° ΔHf products - ° ΔHf reactants ΔHreaction is in kJ or Joules (moles cancel out) Table 2: Standard Enthalpies of Formation Example Calculate the enthalpy change for the following reaction. Use the standard enthalpies of formation listed in Table A-11 on pg 833-834. HCl(g) + NH3(g) NH4Cl(s) ΔHreaction = ΔHf0products - ΔHf0reactants ΔHf0product = (1 mol)(-314.4 kJ/mol) = -314/4 kJ ΔHf0reactants=[(1 mol)(-92.3 kJ/mol)+(1 mol)(-45.9 kJ/mol)] = -138.2 kJ ΔHreaction = (-314.4 kJ) – (-138.2 kJ) -176.2 kJ (exothermic reaction) Additional Practice Calculate the enthalpy change for the following reaction. Use the standard enthalpies of formation listed in Table A-11 on pg 833-834. N2(g) + 3 H2(g) 2 NH3(g) State whether the reaction is exothermic or endothermic. ΔHreaction = ΔH f0products - ΔH f0reactants ΔHf0prod = [(2 mol)(-45.9 kJ/mol) = -91.8 kJ ΔHf0reactants = [(1 mol)(0 kJ/mol) + (3 mol)(0 kJ/mol)] = 0 kJ ΔHreaction = (-91.8 kJ) – (0 kJ) = -91.8 kJ *Reaction is exothermic because ΔH is negative.* Calculating a Reaction’s Change in Enthalpy Sample Prob. E, pg.356 Calculate the change in enthalpy for the reaction below using data from Table 2 on pg 355. 2 H2(g) + 2 CO2(g) 2 H2O(g) + 2 CO(g) State whether the reaction is exothermic or endothermic. ΔHreaction = ΔH f0products - ΔH f0reactants ΔHf0prod = [(2 mol)(-241.8 kJ/mol) + (2 mol)(-110.5 kJ/mol)] = -704.6 kJ ΔHf0reactants = [(2 mol)(0 kJ/mol) + (2 mol)(-393.5 kJ/mol)] = -787 kJ ΔHreaction= (-704.6 kJ) – (-787 kJ) = 82.4 kJ *Reaction is endothermic because ΔH is positive.* Homework Pg. 15 practice workshet MUST show work! Section 10.3 review, pg. 357: #1-5 Concept Review: “Changes in Enthalpy During Chemical Reactions”