Gibbs Free Energy
advertisement

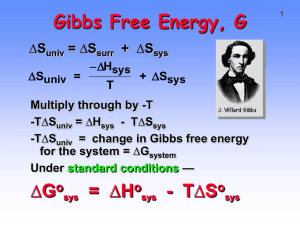
1 Second Law of Thermodynamics As the reaction goes to products our system becomes more disordered and the entropy of our system increases. One driving forces for a chemical reaction is one of the products is a gas. 2 19.3 GIBBS FREE ENERGY ΔSuniverse= ΔSsurroundings+ ΔSsystem = - ΔHsystem / T + ΔSsystem -T ΔSuniverse = ΔHsystem - T ΔSsystem = ΔGsystem ΔG is the change in free energy for the system. ΔGosystem = ΔHosystem - T ΔSosystem (standard state) ΔGorxn from ΔGof and from ΔHorxn and ΔSorxn ΔGorxn= S ΔGfo(products) - S ΔGfo(reactants ΔGorxn = ΔHorxn - T ΔSorxn (Don't forget to change the entropy term from J to kJ) Gibbs Free Energy, G DSuniv = DSsurr + DSsys -D H sys D Suniv = + D Ssys T Multiply through by -T -T ΔSuniv = ΔHsys - T ΔSsys -T ΔSuniv = change in Gibbs free energy for the system = ΔGsystem Under standard conditions — ΔGo = ΔHo - T ΔSo 3 Gibbs Free Energy, G 4 ΔGo = ΔHo - T ΔSo Gibbs free energy change = total energy change for system - energy lost in disordering the system If reaction is exothermic (ΔHo negative) and entropy increases (ΔSo is +), then ΔGo must be negative and reaction product-favored in the standard state. If reaction is endothermic (ΔHo is +), and entropy decreases (ΔSo is -), then ΔGo must be + and reaction is reactant-favored in the standard state. 5 Product-Favored or Reactant-Favored? • The reaction is product-favored if ΔG is negative. • We can see that this is always true if ΔH is negative and ΔS is positive. • If ΔH is positive and ΔS is negative, ΔG is always positive. 6 Product-Favored or Reactant-Favored? • The other two cases are temperature dependent with a positive ΔS favoring spontaneity at high temperature, thus overcoming the positive ΔH, and a negative ΔH favoring spontaneity at a low temperature when ΔS is negative. • Table next slide and Figure 20.8 7 Gibbs Free Energy, G ΔGo = ΔHo - T ΔSo ΔHo ΔSo ΔGo Reaction exo(-) increase(+) - Prod-favored endo(+) decrease(-) + React-favored exo(-) decrease(-) ? T dependent endo(+) increase(+) ? T dependent 8 Figure 20.8 9 Gibbs Free Energy, G ΔGo = ΔHo - T ΔSo Two methods of calculating ΔGo a) Determine ΔHorxn and ΔSorxn and use Gibbs equation. b) Use tabulated values of free energies of formation, ΔGfo. DGorxn = S DGfo (products) - S DGfo (reactants) Calculating D o G rxn 10 Combustion of acetylene C2H2(g) + 5/2 O2(g) --> 2 CO2(g) + H2O(g) Use enthalpies of formation to calculate ΔHorxn = -1238 kJ Use standard molar entropies to calculate ΔSorxn = -97.4 J/K or -0.0974 kJ/K ΔGorxn = -1238 kJ - (298 K)(-0.0974 kJ/K) = -1209 kJ Reaction is product-favored (in the standard state) in spite of negative ΔSorxn. Reaction is “enthalpy driven”. 11 Calculating D o G rxn Is the dissolution of ammonium nitrate productfavored? If so, is it enthalpy- or entropy-driven? NH4NO3(s) + heat NH4NO3(aq) Calculating D NH4NO3(s) + heat 12 o G rxn NH4NO3(aq) From tables of thermodynamic data: ΔHorxn = +25.7 kJ ΔSorxn = +108.7 J/K or +0.1087 kJ/K ΔGorxn = +25.7 kJ - (298 K)(+0.1087 kJ/K) = -6.7 kJ Reaction is product-favored (in the standard state) in spite of negative ΔHorxn. Reaction is “entropy driven”. Calculating DGorxn DGorxn = S DGfo (products) - S DGfo (reactants) 13 Combustion of carbon C(graphite) + O2(g) --> CO2(g) ΔGorxn= ΔGfo(CO2) - [ΔGfo(graph) + ΔGfo(O2)] ΔGorxn = -394.4 kJ - [ 0 + 0 ] Note that free energy of formation of an element in its standard state is 0. ΔGorxn = -394.4 kJ Reaction is product-favored as expected. 14 Free energy and Temperature • If we assume that ΔHo and ΔSo are relatively independent of temperature, we can calculate ΔGo at any particular temperature we choose. ΔGTorxn = ΔHorxn - T ΔSorxn 15 Free Energy and Temperature 2 Fe2O3(s) + 3 C(s) ---> 4 Fe(s) + 3 CO2(g) ΔHorxn = +467.9 kJ ΔSorxn = +560.3 J/K ΔGorxn = +300.8 kJ Reaction is reactant-favored at 298 K At what T does ΔGorxn just change from being (+) to being (-)? When ΔGorxn = 0 = ΔHorxn - T ΔSorxn. DHrxn 467.9 kJ T = = = 835.1 K DSrxn 0.5603 kJ/K 16 Free Energy and Temperature • For the reaction below: Calculate ΔGo at 298.15 K two ways, explain the sign of ΔSo, determine if the reaction in the standard state is product-favored at 298.15 K, determine which term or terms favor spontaneity, and calculate the temperature at which the reaction would first become reactant favored in the standard state. 3 H2(g) + CO(g) ====> CH4(g) + H2O(g) 20.4 THERMODYNAMICS AND THE EQUILIBRIUM CONSTANT 17 At equilibrium, ΔGT = 0, and ΔGTo = -RT lnKT • Figure 20.11, shows the relationship between Q and K, which comes from the concentration dependence of Free Energy. • One cannot calculate a new K by simply changing the T in the equation since ΔGTo is a function of temperature. ΔGTorxn = ΔHorxn - TΔSorxn • A useful combined form of these equations is: ΔGTo = ΔHo - TΔSo = -RT ln KT. 18 Figure 20.11 Thermodynamics and Keq Keq is related to reaction favorability. When ΔGorxn < 0, reaction moves energetically “downhill” ΔGorxn is the change in free energy as reactants convert completely to products. But systems often reach a state of equilibrium in which reactants have not converted completely to products. In this case ΔGrxn is < ΔGorxn , so state with both reactants and products present is more stable than complete conversion. 19 Thermodynamics and Keq 20 Product-favored reaction. 2 NO2 ---> N2O4 ΔGorxn = -4.8 kJ Here ΔGrxn is less than ΔGorxn , so the state with both reactants and products present is more stable than complete conversion. 21 Thermodynamics and Keq Reactant-favored reaction. N2O4 --->2 NO2 ΔGorxn = +4.8 kJ Here ΔGorxn is greater than ΔGrxn , so the state with both reactants and products present is more stable than complete conversion. 22 Thermodynamics and Keq Keq is related to reaction favorability and so to ΔGorxn. The larger the value of ΔGorxn the larger the value of K. o ΔG rxn = - RT lnK where R = 8.31 J/K•mol 23 Thermodynamics and Keq DGorxn = - RT lnK Calculate K for the reaction N2O4 --->2 NO2 ΔGorxn = +4.8 kJ ΔGorxn = +4800 J = - (8.31 J/K)(298 K) ln K lnK = - 4800 J = - 1.94 (8.31 J/K)(298K) K = 0.14 When ΔGorxn > 0, then K < 1 24 THERMODYNAMICS AND THE EQUILIBRIUM CONSTANT • Let us consider the derivation of these equations to further our understanding of them and their interrelationships. • This derivation starts with the definition of G, G = H - TS • At constant temperature, with H = E + PV, and wext = 0,and w = -PdV, we end up with, ΔG = nRT ΔP/P. 25 THERMODYNAMICS AND THE EQUILIBRIUM CONSTANT G = Go + nRT ln a, where a, is the activity, unitless concentration. • For a reaction, we arrive at: ΔGT = ΔGTo + RT • Where Q = K at equilibrium. ln QT 26 DERIVATIONS • Derive the relationships between ΔGTo and KT. • Derive the relationships between ΔHo , KT , and T. 27 THE EQUILIBRIUM CONSTANT • The temperature dependence of K can be calculate using the equation: ΔGTo = ΔHo - T ΔSo = - RT ln KT. • This equation can be written at two temperatures, T1 and T2, and combined to eliminate ΔSo. • This produces the very useful equation: KT2 ln K T1 - D H 1 - 1 = R T 2 T1 28 THE EQUILIBRIUM CONSTANT A plot of ln K vs. 1/T yields a straight line with a slope of - Δ Ho/R. 29 20.5 THERMODYNAMICS AND TIME • The first and second Laws of Thermodynamics cannot be proven, they are laws of experience and tell us the direction of time in any given "picture". 30 Real World Examples of: Chapter 20: Second Law of Thermodynamics “The total entropy of the universe is always increasing” liquid H 2 O(l) vaporization condensation 40.7 kJmol gas -1 -40.7 kJmol -1 H 2 O( g ) 31 Faster molecules can break intermolecular forces like H-bonding to escape a solution 32 Kinetic Molecular Theory of Gases 33 Experiment: Two Florence flasks with H2O, one closed and the other open. This experiment demonstrates: 1. There is sufficient heat in the surroundings to allow liquid water to escape to the atmosphere; 2. Air currents and gas diffusion prevent the gaseous water from making contact with the water surface. 34 The Clausius-Clapeyron equation is a method for obtaining enthalpy of vaporization, at any temperature. DH vap ln Pvap = - C RT 35 In a closed system…Questions 1. Can we change the equilibrium in this system? 2. Is there any reason for wanting to change the equilibrium in this system? 36 Closed system equilibrium H 2 O(l) Kp = H 2 O( g ) PH O 2 Pwater vapor Q < K Reaction favors reactants to products Q > K Reaction favors products to reactants Q = K Reaction is at equilibrium 37 Frost-free freezers Kp = PH O 2 Pwater vapor Q < K favors reactants to products Air in the freezer is warmed then dried. The vapor pressure of ice is 4.579 torr. Warm, desiccated air can remove water vapor. 38 Second Law of Thermodynamics As the reaction goes to products our system becomes more disordered and the entropy of our system increases. One driving forces for a chemical reaction is one of the products is a gas. 39 Gibbs free energy H 2 O(l) H 2 O( g ) DG = Grxn RT ln Q DGrxn = - RT ln K p DGrxn = DH rxn - T DS rxn 44.0kJ - 35.5kJ = 8.58kJ K p = .0313 40 Gibbs free energy DGrxn = 8.58kJ K p = .0313bar DG > 0 reactant favored DG = 0 equilibrium DG < 0 product favored, reaction proceeds to products 41 If Q < K product favored What are the conditions necessary for the spontaneous formation of products? DG = Grxn RT ln Q Q= PH O 2 Pwater vapor A Q of .0313 allows water vapor to evaporate. The atmosphere has a PH2O of .001%-4% water vapor (3%-100% humidity), in the winter Pvap can be as high as 20 torr. 42 Summary Water will Evaporate even though the process takes energy •Kinetic Molecular Theory PH O •Evidence that K p = P water vapor 2 is easily manipulated under normal conditions •Highly ordered H2O(l) has a large DS, this is the main driving force for producing a higher partial pressure of water. 43 As a rule of thumb the rate of an rxn doubles, or triples for every 10 degrees increase in temperature. What factors affect the rate of a reaction? The concentration of the reactants. The more concentrated the faster the rate; Temperature. Usually reactions speed up with increasing temperature; Physical state of reactants; Catalyst (or inhibitor). A catalyst speeds up a reaction, an inhibitor slows it down. 44 Δ,H Ester + H 2 O carboxylic acid + alcohol + Exp Temp K k [L/mols] DK K2/K1 1 288 .0521 2 298 .101 10 1.93 3 308 .184 10 1.82 4 318 .332 10 1.80 Concentrations of the Ester and H2O are held constant, only the temperature changes. 45 Arrhenius equation k = Ae - Ea RT Ea 1 ln k = ln A - R T 46 Testing the “Rule of Thumb” K 2 Ea 1 1 ln = - K1 R T1 T2 Setting K2/K1 = 2, T1 = 273, T2 = 283 Solve for Ea, Ea = 44.5kJmol-1 47 Theoretical Results T1 T2 k2/k1 273 283 2.00 373 383 1.45 473 483 1.26 573 583 1.17 673 683 1.12 773 783 1.09 48 When can relying on the rule be dangerous H 2 ( g ) Cl2 ( g ) 2 HCl heat Cl2 ( g ) 2Cl ( g ) Cl ( g ) H 2 ( g ) HCl ( g ) H ( g ) H ( g ) Cl2 ( g ) HCl ( g ) Cl ( g ) Once the rxn is initiated, no further heat is needed.