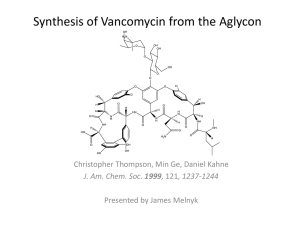
Vancomycin
advertisement

Pharmacokinetics: Monitoring Aminoglycoside and Vancomycin Serum Levels Betty Lee, Pharm.D. Lucile Packard Children’s Hospital July 3, 2012 Outline • Vancomycin pharmacokinetics • Aminoglycosides pharmacokinetics • Guidelines for monitoring aminoglycoside and vancomycin serum levels at LPCH Vancomycin • Volume of distribution: • An average value of 0.7 L/kg or • For patient older than 18 years: V (L) = 0.17 (age in yr) + 0.22 (TBW in kg) + 15 • Eliminated primarily by the renal route; approximately 5% of the dose is metabolized (Vancomycin Cl ~ Clcr) Winter ME. Basic Clinical Pharmacokinetics. 5th ed. Philadelphia: Lippincott Williams and Wilkins, 2010. Vancomycin • t1/2 elimination • • • • Newborns: 6-10 hours Infants & Children 3 months to 4 years: 4 hours Children > 3 years: 2.2 – 3 hours Adults: 5 – 11 hours; significantly prolonged with renal impairment Lexicomp Online, June 2012 Vancomycin • Bactericidal for most gram-positive organisms, except against enterococci • Vancomycin-induced ototoxicity has been primarily reported in patients with vancomycin concentrations > 80 mg/L. • As a single agent, vancomycin is associated with a low incidence of nephrotoxicity; however, when it is combined with aminoglycoside, the incidence may be as high as 30%. Winter ME. Basic Clinical Pharmacokinetics. 5th ed. Philadelphia: Lippincott Williams and Wilkins, 2010. Aminoglycosides • Volume of distribution is ~0.25 L/kg • Pediatric patients younger than 5 years tend to have a larger volume of distribution, declining from an initial value of 0.5 L/kg to adult value of 0.25 L/kg V(children 1-5 yrs) = [(0.5 L/kg) - (age in years x 0.25)] (wt in kg) 5 • Obese patients: V (obese pt) = (0.25 L/kg) (IBW) + 0.1 (TBW-IBW) Winter ME. Basic Clinical Pharmacokinetics. 5th ed. Philadelphia: Lippincott Williams and Wilkins, 2010. Aminoglycosides • Aminoglycosides are eliminated almost entirely by the renal route (Cl = Clcr) • t1/2 elimination (Gentamicin) • • • • Infants < 1 week: 3 – 11.5 hours Infants 1 week to 6 months: 3 – 3.5 hours Adults: 1.5 – 3 hours End-stage renal disease: 36 – 70 hours Winter ME. Basic Clinical Pharmacokinetics. 5th ed. Philadelphia: Lippincott Williams and Wilkins, 2010. Lexicomp Online, June 2012 Aminoglycosides • Dose based on lean weight • If total body weight (TBW) >20% ideal body weight (IBW), use adjusted body weight • Children (1-18 years) IBW calculation (IBW in kg, height in cm): • IBW = (height2 x 1.65)/1000 • IBW = 2.396 x e0.01863 (height in cm) • Adjusted body weight = IBW + [0.4 x (TBW-IBW)] Winter ME. Basic Clinical Pharmacokinetics. 5th ed. Philadelphia: Lippincott Williams and Wilkins, 2010. Aminoglycosides • Bactericidal activity is concentration-dependent • Postantibiotic effect that results in depressed bacterial growth after plasma concentrations have fallen below the MIC • Decreased risk of adaptive resistance • Saturable uptake mechanisms within the renal cortex and inner ear indicate that extended interval dosing may also minimize the likelihood of developing nephrotoxicity and ototoxicity. Fisman DN, Kaye KM. Once-daily dosing of aminoglycoside antibiotics. Infect Dis Clin North Am. 2000 Jun;14(2):475-87. Winter ME. Basic Clinical Pharmacokinetics. 5th ed. Philadelphia: Lippincott Williams and Wilkins, 2010. Once-daily Aminoglycosides • Less intensive monitoring of serum concentrations • Nomogram developed by Nicolau D et al. Antimicrob Agents Chemother 1995; 39:650-655. • Recommends a single level be drawn 6 to 14 hours after the dose • With extended interval dosing there should be no significant accumulation with multiple dosing, therefore, measurements can be obtained after any dose. Nicolau D et al. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob Agents Chemother 1995; 39:650-655. Nicolau D et al. Antimicrob Agents Chemother 1995; 39:650-655 Nicolau D et al. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob Agents Chemother 1995; 39:650-655. Guidelines for Monitoring Aminoglycoside and Vancomycin Serum Levels at LPCH • Order serum levels two times a week for aminoglycoside and at least once a week for vancomycin • Order serum creatinine once or twice per week • For patients undergoing intermittent hemodialysis, vancomycin random levels every three to four days are indicated to ensure serum levels are greater than 10 mcg/mL (or 3 to 4 times above the MIC of the infecting organisms) Guidelines for Monitoring Aminoglycoside and Vancomycin Serum Levels • Vancomycin: • Order a single trough level before the 4th dose of vancomycin • Trough is drawn within 30 minutes before the next dose • Routine peak levels are not necessary for most patients • Consider ordering peak level after the 3rd dose in patients with selected circumstances, such as bacterial meningitis • Peak level is drawn 60 minutes after the end of a 60-min infusion Guidelines for Monitoring Aminoglycoside and Vancomycin Serum Levels • Aminoglycosides: • Order peak after the 3rd dose of aminoglycosides and trough before the 4th dose • Peak is drawn 30 minutes after end of a 30-min infusion • Trough is drawn within 30 minutes before the next dose • If once-daily dosing, order a peak level 60 minutes after end of a 60-min infusion and order a random level at hour 20. Guidelines for Monitoring Aminoglycoside and Vancomycin Serum Levels Aminoglycosides Trough Peak Peak (synergy) Peak (CF patients) Once-daily Peak Once-daily Random (at 18-20 hours) Vancomycin Trough Peak Gentamicin/Tobramycin < 2 mcg/ml 4-10 mcg/ml 3-5 mcg/ml 8-12 mcg/ml 15-25 mcg/ml <1 mcg/ml Amikacin < 10 mcg/ml 20-30 mcg/ml N/A N/A 30-55 mcg/ml <5 mcg/ml *10-20 mcg/ml 25-40 mcg/ml *For patients with meningitis or osteomyelitis, the goal trough levels should be 15-20 mcg/ml. Viscoli C, Dudley, M et al. Serum Concentrations and safety of single daily dosing of amikacin in children undergoing bone marrow transplantation. Journal of Antimicrobial Chemotherapy 1991 27, Suppl. C,113-120. Trujillo H, Robledo J et al. Single daily dose amikacin in paediatric patients with severe Gram-negative infections. Journal of Antimicrobial Chemotherapy 1991 27, Suppl. C, 141-147. Some Useful PK Formulas • K = ln (C1 / C2 ) (t2 – t1) • K = Cl V • t1/2 = 0.693 / K • For steady state, bolus model : Dose = (Css1 ) (V) (1- e-Kτ ) (e-Kt1 ) Winter ME. Basic Clinical Pharmacokinetics. 5th ed. Philadelphia: Lippincott Williams and Wilkins, 2010. Some Useful PK Formulas • CLcr for Children = (K) (Height in cm) (BSA) (ml/min) SCrss (1.73m2) where the K value is based on the infant/child’s age: Age Preterm infants up to 1 year Full-term infants up to 1 year 1-12 years 13-21 years female 13-21 years male Winter ME. Basic Clinical Pharmacokinetics. 5th ed. Philadelphia: Lippincott Williams and Wilkins, 2010. K 0.33 0.45 0.55 0.55 0.70 Some Useful PK Formulas • BSA in m2 = Patient’s weight in kg 70 kg 0.7 (1.73 m2) • CLcr for males = (140 - Age) (Weight) (ml/min) (72) (SCrss) • CLcr for females = (0.85) (140 - Age) (Weight) (ml/min) (72) (SCrss) Winter ME. Basic Clinical Pharmacokinetics. 5th ed. Philadelphia: Lippincott Williams and Wilkins, 2010. Clinical Calculators • Clinical calculator available at LPCH Intranet: Lane Library Specialty Portals Pharmacy-Calculators Drug Levels-Vancomycin & Aminoglycoside Pharmacokinetics • http://medcalc.com.laneproxy.stanford.edu/pk/ • Other calculators available at Pharmacy Network: Pharmacy Network Pharmacokinetic Monitoring • • CF Kinetics - by Dr. Carlos Milla NICU Drug Kinetics - by Dr. William Benitz Additional Information • Area Under the Curve (AUC) = area under the plasma drug concentration vs. time curve • AUC = dose administered/drug clearance • AUC (mg.hr/L)= C0 = initial concentration (mg/L) k elimination rate constant (hr-1) • Gentamicin and Tobramycin: • AUC24 = 70 – 100 mg.hr/L • Cystic fibrosis patients: tobramycin AUC24 ~ 100 to 125 Prescott WA Jr, Nagel JL. Extended-interval once-daily dosing of aminoglycosides in adult and pediatric patients with cystic fibrosis. Pharmacotherapy 2010 Jan;30(1):95-108. CF Kinetics - by Dr. Carlos Milla NICU - Drug Kinetics Additional Information • Vancomycin—some oncology or ICU patients may need up to q 6 hour dosing • Never give aminoglycoside more frequent than q 8 hour • Aminoglycoside—if long term therapy, need hearing test References • Michael E. Winter. Basic Clinical Pharmacokinetics. 5th edition. Lippincott Williams & Wilkins, 2010. • Nicolau D et al. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob Agents Chemother 1995; 39:650-655. References • Rybak M, Lomaestro B, Rotschafer JC et al. Therapeutic monitoring of Vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health-Syst Pharm 2009;66:82-98. • Prescott WA Jr, Nagel JL. Extended-interval once-daily dosing of aminoglycosides in adult and pediatric patients with cystic fibrosis. Pharmacotherapy 2010 Jan;30(1):95108.