Drugs in pregnancy
advertisement
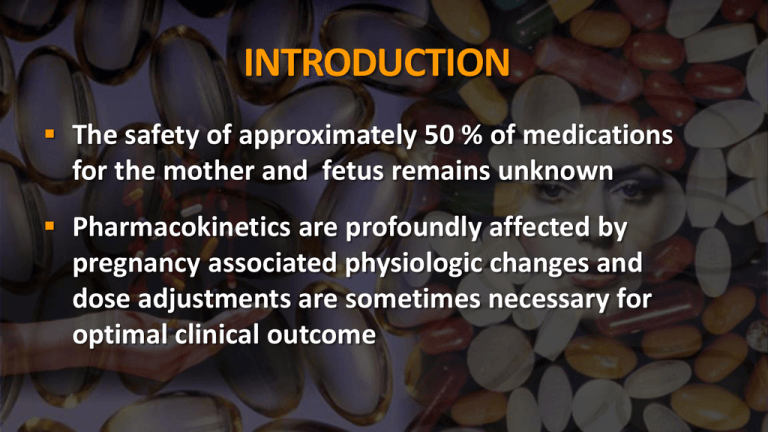
INTRODUCTION The safety of approximately 50 % of medications for the mother and fetus remains unknown Pharmacokinetics are profoundly affected by pregnancy associated physiologic changes and dose adjustments are sometimes necessary for optimal clinical outcome Teratogens A substance, organism, physical agents or deficiency state capable of inducing abnormal structure or function such as: Gross structural abnormalities Functional deficiencies Intrauterine growth restriction Behavioral aberrations Demise Current Categories for Drug Use in Pregnancy Category A : Adequate, well-controlled studies in pregnant women have not shown an increased risk of fetal abnormalities. Examples: Magnesium sulphate Levotyroxine vitamins Current Categories for Drug Use in Pregnancy Category B : Animal studies have revealed no evidence of harm to the fetus, however, there are no adequate and wellcontrolled studies in pregnant women. or Animal studies have shown an adverse effect, but adequate and well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus Examples: Amoxiciliin Amoxicillin + Clavulanic acid Cefotaxime Methyl dopa Metronidazole Erythromycin Current Categories for Drug Use in Pregnancy Category C: Animal studies have shown an adverse effect and there are no adequate and well-controlled studies in pregnant women. or No animal studies have been conducted and there are no adequate and well-controlled studies in pregnant women Examples: Diclofenac Rifampicin Fluoroquinolones Aminoglycosides Glyburide Beta blocker Calcium channel blocker Current Categories for Drug Use in Pregnancy Category D: Studies, adequate well-controlled or observational, in pregnant women have demonstrated a risk to the fetus. However, the benefits of therapy may outweigh the potential harm Examples: Tetracyclines Phenytoin Valproic acid Carbamazepine ACE inhibitors Litium Current Categories for Drug Use in Pregnancy Category X: Studies, adequate well-controlled or observational, in animals or pregnant women have demonstrated positive evidence of fetal abnormalities. The use of the product is contraindicated in women who are or may become pregnant. Examples: Thalidomide Oral contraceptive pills Misoprostol DRUGS USED COMMONLY IN PREGNANCY ANTIBIOTICS: Cephalosporins Fluoroquinolones Macrolides Aminoglycosides Miscellaneous CEPHALOSPORINS (Eg: Ceftriaxone, Cefixime, Cefotaxime) Category B in pregnancy Cross the placenta during pregnancy Some reports of increased anomalies with specific cephalosporins (cefaclor, cephalexin, cephradrine) Primarily cardiac and oral cleft defects Lactation Excreted into breastmilk in low concentrations Considered compatible with breastfeeding FLUOROQUINOLONES (Eg: Ciprofloxacin, Norfloxacin) Pregnancy Category C Not recommended in pregnancy Cartilage damage in animals Safer alternatives usually exist Lactation Excreted into breastmilk Limited human data compatible with breastfeeding MACROLIDES (Eg: Azithromycin, Clarithromycin, Erythromycin) Pregnancy Categories B/C/B Cross the placenta in low amounts Limited data with azithromycin and clarithromycin Lactation Erythromycin compatible Others probably compatible AMINOGLYCOSIDES (Gentamicin, Amikacin) Pregnancy Category C Rapidly cross placenta Enter amniotic fluid through fetal circulation Lactation Compatible with breastfeeding Not absorbed through GI tract Tetracyclines (doxycycline, minocycline, tetracycline) Pregnancy Category D Can cause problems with teeth and bone and other defects/effects Have been linked to maternal liver toxicity Lactation Compatible with breastfeeding Serum levels in infants undetectable MISCELLANEOUS ANTIBIOTICS Clindamycin Pregnancy Category B, commonly used Lactation – Compatible Metronidazole Pregnancy Category B, carcinogenic in animals, avoid in 1st trimester if possible Lactation – hold feeds for 12-24hrs afterward MISCELLANEOUS ANTIBIOTICS Vancomycin Pregnancy Category B, compatible Lactation – likely compatible, not absorbed Nitrofurantoin Pregnancy Category B, possible hemolytic anemia with use at term Lactation – Compatible, avoid with G-6-PD deficiency MISCELLANEOUS ANTIBIOTICS Trimethoprim Pregnancy Category C, potentially problematic early in pregnancy Lactation – Compatible as combination drug Antivirals (acyclovir, famciclovir, valacyclovir) Pregnancy Category B Acyclovir and valacyclovir readily cross the placenta Can be used for HSV treatment and suppression Lactation Acyclovir and valacyclovir are compatible Famciclovir should be avoided Antiretrovirals/NRTI (abacavir, didanosine (ddI), emtricitabine (FTC)) Pregnancy Categories C/B/B Maternal benefit usually outweighs fetal risk Cross the placenta Limited data with each do not show increased risk of anomalies Didanosine has been associated with severe lactic acidosis w/ or w/o pancreatitis Antiretrovirals/NRTI (lamuvidine (3TC), stavudine (d4T)) Pregnancy Category C Maternal benefit usually outweighs fetal risk Cross the placenta by simple diffusion Data with lamivudine show no increased risk of anomalies Stavudine has been associated with severe lactic acidosis w/ or w/o pancreatitis All NRTIs have been possibly linked to mitochondrial dysfunction postnatally Antifungals/Azoles (fluconazole, itraconazole, ketoconazole, posaconazole, voriconazole) Pregnancy Categories C/C/C/D Likely cross placenta Fluconazole > 400mg/day seems to be associated with cranio-facial abnormalities Itraconazole appears to have low risk Ketoconazole can impair testosterone and cortisol synthesis No data in humans is available for voriconazole, increased risk in animals Antifungals/Azoles (fluconazole, itraconazole, ketoconazole, posaconazole, voriconazole) Lactation Fluconazole is compatible Itraconazole could concentrate in milk and body tissues, not recommended Ketoconazole is compatible No data with voriconazole, not recommended Migraine Headache Therapy Triptans Ergots Caffeine Triptans (5-HT1 agonists) Pregnancy Category C Limited human data exists, sumatriptan has been associated with VSDs in several cases No data available in humans for almotriptan, eletriptan, frovatriptan, or zolmitriptan Limited human data exists with naratriptan and rizatriptan, although animal data indicates moderate risks Pregnancy registry available for exposures Triptans (5-HT1 agonists) Lactation Cross into breastmilk and may concentrate No reports of human lactation with almotriptan, frovotriptan, naratriptan, rizatriptan, or zolmitriptan Sumatriptan is compatible Eletriptan is likely compatible with low concentrations Ergots (Dihydroergotamine, ergotamine) Pregnancy Category X Oxytocic properties could cause IUGR by vascular disruption or increased uterine tone Early exposure appears safe, not teratogens Chronic exposure is contraindicated Lactation Contraindicated ANTICONVULSANTS HYDANTOIN AGENTS Category D Hydantoin agents (Phenytoin) are teratogens long recognised for constellation of congenital anomalies known as fetal hydantoin syndrome The syndrome consists of craniofacial abnormalities , mental deficiency , hypoplasia of phalanges ANTICONVULSANTS CARBAMAZEPINE: Category D Was considered relatively safe for use during pregnancy but recent FDA reports suggest increased risk of neural tube defects with carbamazepine too and a pattern of malformations similar to phenytoin Increases the risk facial dysmorphology, neural tube defects, cardiovascular defects, and urinary tract defects. ANTICONVULSANTS VALPROIC ACID: Category D It is commonly used for petit mal seizures 1 to 2 % risk of neural tube defects with use in pregnancy atrial septal defect, cleft palate, hypospadias, polydactyly, and craniosynostosis. Antidepressants Recent publications have implicated some of the SSRIs administered in the last trimester with postnatal neurobehavioral effects that are transient and whose long-term effects have not been determined. Firsttrimester exposures to some SSRIs have been reported to increase the risk of some congenital malformations, predominantly congenital heart disease. The results have not been consistent, but warnings have been issued. Antituberculous therapy Isoniazid and paraaminosalicylic acid have an increased risk for some CNS abnormalities. Corticosteroids High exposures administered systemically have a low risk for cleft palate in some studies, but the epidemiologic studies are not consistent. Methimazole Aplasia cutis has been reported to be increased in mothers administered this drug during pregnancy* Warfarin and warfarin derivatives Early exposure during pregnancy can result in nasal hypoplasia, stippling of secondary epiphysis, intrauterine growth restriction. Central nervous system malformations can occur in late pregnancy exposure because of bleeding. NSAIDs and aspirin Aspirin has been used for years in patients requiring NSAID therapy during pregnancy. Salicylates readily cross the placenta, but a 2002 meta-analysis did not find evidence of an overall increase in risk of congenital defects associated with first trimester use of aspirin .Although some case-control studies have reported associations between human congenital malformations and aspirin use early in gestation, no consistent adverse outcome attributable to drug use has been observed. The NSAIDs and salicylates are considered by the FDA as category C drugs in terms of the risks associated with their use during the first two trimesters .A number of the NSAIDs are labeled as category D drugs from week 30 onwards, and high-dose aspirin is considered a category D medication during the third trimester However, third trimester use of these agents may pose greater risk. The inhibition of prostaglandin synthesis by NSAIDs or by high-dose aspirin therapy in the third trimester has the potential for causing premature closure of the ductus arteriosus; indomethacin and ibuprofen appear to have much stronger ductal effects than aspirin .High-dose aspirin near delivery may increase the risk of fetal or neonatal bleeding or bruising although data are inconsistent PROSTAGLANDINS They are synthesised from essential fatty acids PGF2a promotes myometrial contractility , is produced mainly from decidua PGE2 helps cervical maturation / ripening , is mainly produced from amnion They also sensitise myometrium to oxytocin Commonly used for induction of labour PROSTAGLANDINS PGE1 – Misoprostol: (Category X) PGE1 promotes cervical ripening and myometrial contractility is increased Transvaginally used for induction of labour Failure of induction is less Can be used per rectally /orally also Incidence of tachysystole is high and thus should not be used in cases with previous ceasarean birth ANTIHYPERTENSIVES METHYL DOPA: Category B It is the drug of first choice in pregnancy Has central and peripheral anti adrenergic action Safe for both mother and fetus Postural hypotension is a common side effect May be given orally or i.v Doses start from 25o mg bd to 500 mg four times a day ANTIHYPERTENSIVES NIFEDIPINE: Cause direct arteriolar vasodilatation by inhibition of slow calcium channels Flushing , hypotension , headache , tachycardia are side effects noted ANTIHYPERTENSIVES LABETALOL: Has combined alpha and beta adrenergic blocking actions Can be used orally and as iv infusion Efficacy and safety appears to be equal to methyl dopa Dose is 100 mg twice a day ANTIHYPERTENSIVES ACE INHIBITORS: Category C or D Not used in pregnancy as studies show increased risk of oligohydramnios , neonatal anuria , renal anomalies and nephrotoxicity when used in 2 nd and 3 rd trimesters Thus considered human teratogens ANTIHYPERTENSIVES SODIUM NITROPRUSSIDE: It is used to treat serious , life threatening hypertension Animal studies have shown fetal cyanide toxicity but human studies have not proved the same Nonetheless , it is avoided in preganancy and is only used as last resort ANTIHYPERTENSIVES MAGNESIUM SULPHATE: Category A Mechanism of action : It decreases acetycholine release from nerve endings and reduces motor end plate sensitivity to acetylcholine It blocks calcium channels and causes vasodilation Can be given by Pritchard regime or Zuspan regime Pritchard Regime: 4 gm iv slowly followed by 5 gm in each buttock deep im -- loading dose 5 gm deep im 4 hourly in alternate buttock Indications: In eclampsia , as an anticonvulsant As a tocolytic Contraindications In patients with renal impairment Dosage: For I.V infusion , 50% solution must be diluted to 20 % before administration 50% solution (undiluted) is given for intramuscular injections It is relatively safe . Muscular paresis , respiratory failure and renal effects on mother are known side effects Thus deep tendon reflexes, respiratory rate and urine output monitoring is essential in a patient receiving Magnesium Sulpahate Has no harmful effects on fetus though neonatal respiratory depression may be seen TOCOLYTICS BETAMIMETICS:( Terbutaline , Isoxsuprine) Category C Mechanism of action: They activate intracellular enzymes and reduce intracellular free calcium which leads to reduced interaction of actin and myosin Dosage: Terbutaline can be subcutaneously 0.25 mg 6 hourly or orally 0.5 mg 6 hourly Isoxsuprine is given either as intravenous infusion drip or intramuscularly(10mg 6 hourly) or orally (10 mg 6/8 hourly) Contraindications : Cardiac arrhythmias Poorly controlled diabetes mellitus Poorly controlled thyroid disorders Maternal side effects are headache , palpitations , pulmonary edema , hyperglycemia and hypotension Fetal tachycardia , heart failure may be seen INDOMETHACIN AND CALCIUM CHANNEL BLOCKERS : They are also used commonly for tocolysis Indomethacin may cause gastric disturbances in mother Calcium channel blockers may cause headache , flushing Both cause no known fetal harm OXYTOCIN Mechanism of action: It acts through calcium channels to initiate myometrial contractions Also stimulate amniotic and decidual prostaglandin production OXYTOCIN Routes of administration: Can be given intramuscularly or by controlled intravenous infusion It is also available as nasal solution , buccal tablets OXYTOCIN Indications: Induction of labour Augmentation of labour In active management of third stage, as an alternative to methergin To control postpartum hemorrhage OXYTOCIN Contraindications: Obstructed labour Malpresentations Contracted pelvis History of previous Caesarean section/hysterotomy (relative contraindication) OXYTOCIN Maternal side effects: Uterine hyperstimulation (sometimes rupture) Water intoxication due to its antidiuretic effect Hypotension OXYTOCIN Fetal side effects: Fetal distress , fetal hypoxia or even fetal death may occur due to hyperstimulation ERGOT DERIVATIVES METHERGIN: (Category X) Mechanism of action: Acts directly on myometrium and cause tetanic uterine contractions Route of administration: Parenterally – 0.2 mg ampoules available Orally – 0.5 or 1 mg tablets available ERGOT DERIVATIVES Indications: Therapeutic: To stop atonic uterine bleeding following delivery or abortion Prophylactic : Should be only used in second stage of labour after delivery of anterior shoulder or following delivery of baby ERGOT DERIVATIVES Contraindications: In cardiac diseases Rh negative pregnancies Severe pre-eclampsia/eclampsia IRON DEXTRAN It is intramuscularly used iron preparation for treatment of iron deficiency anemia 1 ml of iron dextran contains 50 mg elemental iron Oral iron to be stopped 24 hour before therapy to avoid reactions IRON DEXTRAN Mode of administration: Dose to be given is initially calculated Initial test dose is given This is followed by daily or alternate day injections given deep im by Z technique IRON DEXTRAN Drawbacks: Injections are painful May cause staining of skin Allergic reactions , though rare , may occur Abscess formation over injection site may occur IRON DEXTRAN Indications: Iron deficiency anemia , when oral iron therapy is unsatisfactory or not tolerated Contraindications: Anemia other than iron deficiency Hypersensitivity to the product Ionizing radiationRadiation Ionizing radiationRadiation exposure above a threshold of 20 rad (0.2 Gy) can increase the risk for some fetal effects such as microcephaly or growth retardation, but the threshold for mental retardation is higher. Some common teratogenic medications include: Angiotensin converting enzyme inhibitors. Anticonvulsant agents Antineoplastic agents Thalidomide, retinoic acid, methylene blue, misoprostol, penicillamine, fluconazole, lit hium, isotretinoin, and acitretin Some common teratogenic medications include: Retinoic acid is highly teratogenic in the first trimester of pregnancy, leading to spontaneous abortions and fetal malformations, including microcephaly and cardiac anomalies . At doses of only several times the RDA , many animal models as well as human studies have shown high incidence of birth defects in mothers who ingested therapeutic doses of retinoic acid for dermatological uses . A safe upper limit for vitamin A intake has been recognized at about 800 to 10,000 IU/day . Acitretin should not be used by women who want to become pregnant as conception is contraindicated for at least three years after discontinuation. Some common teratogenic medications include: Androgenic agents, such as testosterone or danazol, do not cause malformation, but can virilize a female fetus. Cocaine induced vasoconstriction of uterine vessels is one mechanism for fetal damage from this substance . Infants whose mothers consume alcohol during pregnancy can have fetal alcohol effects (FAE), alcoholrelated birth defects (ARBD), fetal alcohol syndrome, or they may be normal. Some common teratogenic medications include: Folicacid antagonists(eg, trimethoprim, triamterene, car bamazepine, phenytoin, phenobarbital, primidone, met hotrexate) increase the risk of neural-tube defects and possibly cardiovascular defects, oral clefts, and urinary tract defects and placenta-mediated adverse pregnancy outcomes, including preeclampsia, placental abruption, fetal growth restriction, and fetal death . Specific information on the fetal and neonatal risks of maternal drug ingestion during pregnancy and lactation are available from several resources , including: www.perinatology.com/exposures/druglist.htm www.reprotox.org/ (requires subscription) www.OTISpregnancy.org file://depts.washington.edu/terisweb/teris/ (requires subscription) www.motherisk.org/women/drugs.jsp UpToDate drug information database Antibiotics in Pregnancy Penicillins Erythromycin Cephalosporins Nitrofurantoin Metronidazole Trimethoprim Sulpha drugs Chloramphenicol Tetracycline Gentamicin A A A A B2 B3 C A D D Anti-malarial drugs for Pregnancy Chloroquine A Quinine D Paludrine B2 Maloprim, Daroprim B3 Larium B3 Fansidar D Doxycycline D HAART drugs for Pregnancy AZT B3 Lamivudine B3 Nevirapine B3 3TC B3 Abacavir B3 Anti-emetics for Pregnancy Pyridoxine A Diphenhydramine A Metoclopromide A Hyoscine B2 Ondansetron B1 Promethazine C Prochlorperazine C Antihypertensive drugs in Pregnancy Aldomet A Hydralazine C Beta blockers C Ca channel blockers C Thiazides C ACE Inhibitors D ↑risk of CNS & CHD defects 3- Analgesic Drugs for Pregnancy Paracetamol A Codeine A Aspirin C Narcotics C NSAIDs C Have the potential to cause in utero closure of the ductus arteriosus >34w THANK YOU