Navigating Taxane Managment - Advanced Studies in Nursing
advertisement

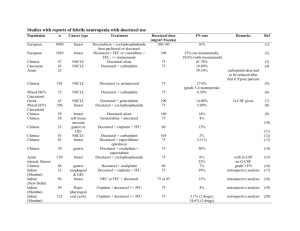
Treatment of Hormone-refractory Prostate Cancer Sandra Kurtin, RN, MS, AOCN, ANP-C Hematology/Oncology Nurse Practitioner Arizona Cancer Center Clinical Assistant Professor of Nursing Clinical Assistant Professor of Medicine University of Arizona Tucson, AZ NCCN Guidelines: Treatment of Hormone-refractory Prostate Cancer • Docetaxel-based regimens have been shown to confer survival benefit in two phase 3 studies • SWOG 9916 compared docetaxel plus estramustine with mitoxantrone plus prednisone; median survival for the docetaxel arm was 18 months vs 15 months for the mitoxantrone arm (P = .01) • TAX 327 compared 2 docetaxel schedules (weekly and every 3 weeks) with mitoxantrone and prednisone; median survival for the every-3-weeks docetaxel arm was 18.9 months vs 16.5 months for the mitoxantrone arm (P = .009) • Mitoxantrone with prednisone has been shown to provide palliative benefit in patients with painful bony metastases from castration-recurrent prostate cancer; however, its efficacy as second-line therapy after docetaxel has not been determined National Comprehensive Cancer Network (NCCN) Practice Guidelines in Oncology , v2, 2007. http://www.nccn.org/professionals/physician_gls/PDF/prostate.pdf. NCCN Guidelines: Treatment of Hormone-refractory Prostate Cancer (cont’d) • Docetaxel-based regimens are now the standard of care for first-line treatment in this group of patients • The US Food and Drug Administration has approved docetaxel for injection in combination with prednisone for the treatment of castration-recurrent metastatic (hormone-refractory, androgen-independent) prostate cancer NCCN Practice Guidelines in Oncology , v2, 2007. http://www.nccn.org/professionals/physician_gls/PDF/prostate.pdf. Docetaxel Schedules and Toxicities in Hormone-refractory Prostate Cancer Docetaxel* 30 mg/m2 weekly (n = 330) Docetaxel* 100 mg/m2 every 3 weeks (n = 332) Neutropenia (≥ grade 3) 2% 32% Anemia (≥ grade 3) 5% 5% Neuropathy 24% 30% Fatigue 49% 53% Nail changes 37% 30% Tearing 21% 10% Stomatitis 17% 20% Adverse Event (% of patients) *In combination with prednisone; both regimens tested against mitoxantrone. Tannock IF et al. N Engl J Med. 2004;351:1502-1512. Taxane-associated Hypersensitivity • Incidence: ≈40% of all patients experience minor reactions (≈3% life-threatening) despite premedications • Overall incidence • Paclitaxel: 8% to 45% • Docetaxel: 5% to 20% • Onset: Immediate to within 5–10 minutes • Signs and symptoms: “feeling funny”, flushing, lightheadedness, pruritus, back pain, tightness in chest/neck, bronchospasm, angioedema, hypotension, hypertension Lenz HJ. Oncologist. 2007;12:601-609. Hypersensitivity Reactions in the Outpatient Oncology Setting • Virtually all chemotherapeutic agents have the potential to initiate a hypersensitivity reaction • Reactions often happen in a matter of seconds and without cutaneous symptoms • Anaphylaxis occurs as a continuum • Symptoms are not immediately life-threatening but may progress rapidly if not treated promptly Clinical Dilemma: Balancing the Risk and Benefit • Discontinuation or delay of potentially curative therapy • Delivery of substandard therapy • Patient safety is the primary concern • Death most commonly results from intractable bronchospasm, asphyxiation from upper airway edema, or cardiovascular collapse (vascular leak) Prevention Is the Best Strategy • Know the patient • Primary diagnosis: bulky disease, pulmonary disease, neuroendocrine tumors, carcinoid • Comorbidities: congestive heart failure, reactive airways disease, chronic obstructive pulmonary disease (COPD), diabetes, atrial fibrillation, hepatic or renal disease (decreased metabolism/excretion) • Concomitant mediations: prednisone, cardiac medications • Previous treatment: increased risk for reactions • History of hypersensitivity to medications • Know the drug • Risk is increased with higher doses, rapid infusion rate, drugs derived from bacteria (L-asparaginase) or given as crude preparations (phase I drugs), monoclonal antibodies, taxanes, and platinum compounds • Know the interventions • Adopt a protocol for management of anaphylactoid/anaphylactic reactions Khoukaz T. Semin Oncol Nurs. 2006;22:20-27. Common Agents for Prevention of Hypersensitivity With Taxanes Class Agents and Dosing H1 antagonist Diphenhydramine 50 mg IV H2 antagonist Cimetidine 300 mg IV 30 min prior Ranitidine 50 mg IV 30 min prior Famotidine 20 mg IV 30 min prior Steroid Paclitaxel: Dexamethasone 20 mg IV 30 min prior or 8–20 mg orally 12 and 6 h prior Docetaxel: Every 3 weeks: 8 mg (orally 1 day prior, twice daily 3 days) Weekly: 4–8 mg (1 h prior) For prostate cancer: dexamethasone 8mg PO 12, 6 and 1 hour prior to infusion. Hainsworth JD. Oncologist. 2004;9:538-545; Kwon JS et al. Gynecol Oncol. 2002;84:420-425; Markman M et al. J Cancer Res Clin Oncol. 1999;125:427-429. Treatment of Hypersensitivity • Patient exhibits signs and symptoms of hypersensitivity • Stop infusion • Institute ABCs—airway, breathing, circulation • Assess—vital signs, pulse oximeter, patient complaints • Notify provider • If mild to moderate • Treat symptoms • Provide ongoing assessment until provider arrives • Administer additional premedications if indicated • Consider rechallenging the patient with a 50% reduction in infusion rate with gradual titration as tolerated • Monitor closely during infusion and for 1 hour following infusion • Premedicate for future infusions and consider steroid taper Khoukaz T. Semin Oncol Nurs. 2006;22:20-27. Treatment of Hypersensitivity (cont’d) • If moderate to severe with airway symptoms • Initiate emergency services based on institution policy • Administer diphenhydramine 25–50 mg IV, hydrocortisone 100 mg IV • If patient exhibits progressive symptoms with airway compromise • Give epinephrine (1:1000) 0.3–0.5 mL subcutaneous, repeat every 5–15 minutes • If no improvement, give epinephrine (1:10,000 solution in 10mL syringe) 1 mg over 5 minutes, maximum of 3 doses • Patient will require monitoring overnight • If continued airway symptoms, 2 puffs albuterol metered-dose inhaler • If O2 saturation <90%, administer 100% O2, use nonrebreather for patients with COPD/CO2 retention Khoukaz T. Semin Oncol Nurs. 2006;22:20-27. Steroid-induced Hyperglycemia in the Cancer Patient • Steroids induce a state of relative insulin resistance • May promote glucose production in the liver • Reduce binding of insulin to the insulin receptor on cells • Decrease insulin secretion from the islet cell • Insulin resistance causes primarily postprandial hyperglycemia • Patients with cancer who are receiving steroids as a part of their therapy may develop treatment-related hyperglycemia or overt diabetes • Patients with existing diabetes may require modification of their diabetes medication regimen • Steroid-induced diabetes is related to the dose of steroids used but not the type Oyer DS et al. J Support Oncol. 2006;4:479–483. Steroid-induced Hyperglycemia in the Cancer Patient (cont’d) • Common symptoms associated with hyperglycemia • Polydipsia (excessive thirst) • Polyphagia (excessive hunger) • Polyuria (excessive urination) • Agitation, irritability • Fatigue • Nausea, vomiting • Dry mouth • Visual disturbances