anoro ellipta
advertisement

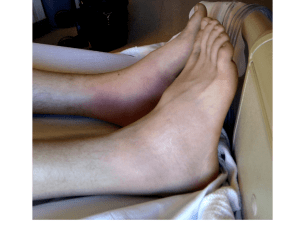
™ Ellipta Anoro umeclidinium/vilanterol Manufacturer: GlaxoSmithKline FDA Approval Date: 12/18/2013 Anoro Ellipta™ - Umeclidinium/Vilanterol Clinical Application • Indications: • Maintenance treatment of airflow obstruction in patients with COPD • Place in therapy: • Anoro Ellipta is the first fixed-dose combination of a LABA and anticholinergic agent Anoro Ellipta™ - Umeclidinium/Vilanterol Clinical Application • Contraindications: • Severe hypersensitivity to milk protein or any ingredients • Black Box warnings • LABAs increase the risk of asthma-related death Anoro Ellipta™ - Umeclidinium/Vilanterol Clinical Application • Warnings and Precautions • Do not initiate in acutely deteriorating COPD or to treat acute symptoms • Do not use with other LABAs • Discontinue if paradoxical bronchospasm occurs • Use with caution in cardiovascular disorders, convulsive disorders, thyrotoxicosis, diabetes, ketoacidosis, and narrow-angle glaucoma • Worsening of urinary retention may occur • Monitor for hypokalemia and hyperglycemia Anoro Ellipta™ - Umeclidinium/Vilanterol Clinical Application • Pregnancy: • Category C • Lactation: • It is unknown if Anoro Ellipta is excreted in breast milk so use with caution Anoro Ellipta™ - Umeclidinium/Vilanterol Drug Facts • Pharmacology: • Umeclidinium (anticholinergic): inhibits M3 recepto at the smooth muscle leading to bronchodilation. Also known as LongActing Muscarinic Antagonist (LAMA) • Vilanterol (LABA): stimulation of intracellular adenyl cyclase, which catalyzes ATP to cAMP. Increased cAMP causes relaxation of bronchial smooth muscle Anoro Ellipta™ - Umeclidinium/Vilanterol Drug Facts Absorption • Cmax 5-15 minutes. • Steady-state was achieved in 14 days Distribution • Umeclidinium: VD = 86L, protein binding 89% • Vilanterol: VD = 165L, protein binding 94% Metabolism • Umeclidinium: CYP2D6 • Vilanterol: CYP3A4 Elimination • T1/2 = 11 hours • Umeclidinium: 92% feces, 1% urine • Vilanterol: 70% urine, 30% feces Anoro Ellipta™ - Umeclidinium/Vilanterol Drug Interactions • Drug Interactions – Object Drugs: • Non-potassium-sparing diuretic induced hypokalemia is () by vilanterol • Anticholinergic medications () anticholinergic effects of umeclidinium Anoro Ellipta™ - Umeclidinium/Vilanterol Drug Interactions • Drug Interactions – Precipitant Drugs: • CYP3A4 inhibitors () levels of vilanterol • MAOI and TCAs () QTc interval when used with vilanterol • Beta blockers () effects of vilanterol Anoro Ellipta™ - Umeclidinium/Vilanterol Adverse Effects Adverse Effect Pharyngitis Sinusitis Lower respiratory tract infection Constipation Diarrhea Pain in extremity Muscle spasms Neck pain Chest pain (Anoro Ellipta%)[Placebo%] (2%) [<1%] (1%) [<1%] (<1%)[1%] (1%)[<1%] (2%)[1%] (2%)[1%] (1%)[<1%] (1%)[<1%] (1%)[<1%] Anoro Ellipta™ - Umeclidinium/Vilanterol Monitoring Parameters • Efficacy Monitoring: • Improvement in lung function • Toxicity Monitoring: • Cardiac monitoring is recommended in cases of overdosages Anoro Ellipta™ - Umeclidinium/Vilanterol Monitoring Parameters • Toxicity Signs/Symptoms: • Umeclidinium: anticholinergic signs and symptoms • Vilanterol: angina, hypertension or hypotension, tachycardia, arrhythmias, nervousness, headache, tremor, seizures, muscle cramps, dry mouth, palpitation, nausea, dizziness, fatigue, malaise, insomnia, hyperglycemia, hypokalemia, metabolic acidosis Anoro Ellipta™ - Umeclidinium/Vilanterol Prescription Information • Dosing: • Uneclidinium/vilanterol 62.5 mcg/25 mcg as 1 inhalation once daily • Cost: – Not available at time of review Anoro Ellipta™ - Umeclidinium/Vilanterol Literature Review • Objective: to examine the efficacy and safety of umeclidinium/vilanterol (UME/VI) compared with UME and VI monotherapies in COPD • Treatment • • • • UMEC/VI 62.5/25 mcg UME 62.5 mcg VI 25 mcg Placebo • Primary endpoint: FEV1 on Day 169 Donohue JF, et al. Respir Med. 2013;107:1538-46 Anoro Ellipta™ - Umeclidinium/Vilanterol Literature Review • Statistics • 273 patients in each active arm and 183 patients in the placebo arm would have 90% power to detect a 1-unit difference between treatments. • This also gae >99% to detect a 0.1 L difference in trough FEV1 • Assuming a withdrawal rate of 30%, 399 patients were needed for the active arms and 266 patients were needed for placebo Donohue JF, et al. Respir Med. 2013;107:1538-46 Anoro Ellipta™ - Umeclidinium/Vilanterol Literature Review Patient Demographics Donohue JF, et al. Respir Med. 2013;107:1538-46 Anoro Ellipta™ - Umeclidinium/Vilanterol Literature Review Primary Endpoint Donohue JF, et al. Respir Med. 2013;107:1538-46 Anoro Ellipta™ - Umeclidinium/Vilanterol Literature Review Adverse Effects Donohue JF, et al. Respir Med. 2013;107:1538-46 Anoro Ellipta™ - Umeclidinium/Vilanterol Literature Review • Study Conclusions • Once-daily umeclidinium/vilanterol significantly improved lung function and symptoms in patients with COPD compared to monotherapy or placebo Donohue JF, et al. Respir Med. 2013;107:1538-46 Anoro Ellipta™ - Umeclidinium/Vilanterol Literature Review • Study Limitations • No head-to-head comparisons of LAMA or LABA monotherapy • No approved LAMA/LABA combination therapy for comparison • Concomitant inhaled corticosteroid or bronchodilator therapies were allowed Donohue JF, et al. Respir Med. 2013;107:1538-46 Anoro Ellipta™ - Umeclidinium/Vilanterol Summary • First approved combination of a long actinganticholinergic (umeclidinium) and a LABA (vilanterol) • Indicated for the maintenance treatment of COPD. NOT approved for asthma. • It has one black box warning: LABAs increase the risk of asthma-related death • Well tolerated. Side effects include; Pharyngitis, diarrhea, and pain in the extremities Anoro Ellipta™ - Umeclidinium/Vilanterol References • Anoro Ellipta [package insert]. GlaxoSmithKline. Dec. 2013. • Donohue JF, et al. Respir Med. 2013;107:1538-46