Medical Oncology - University of Toronto
advertisement

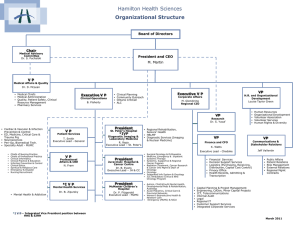
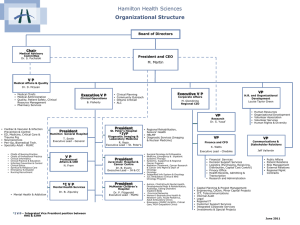
University of Toronto Province-Wide Oncology Rounds May 18, 2012 The EBCTCG Overview: Is it still relevant in 2012? By Dr. Kathleen I. Pritchard Department Division Director, Medical Oncology Professor, Department of Medicine Faculty of Medicine, University of Toronto Medical Oncology 4/8/2015 The Oxford Overview Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Medical Oncology EBCTCG OVERVIEW K. Albain, S. Anderson, R. Arriagada, W. Barlow, J. Bergh, J. Bliss, M. Buyse, D. Cameron, M. Clarke, A. Coates, R. Collins, J. Costantino, J. Cuzick, S. Darby, N. Davidson, C. Davies, A. Di Leo, M. Dowsett, M. Ewertz, R. Gelber, C. Geyer, J. Godwin, A. Goldhirsch, R. Gray, D. Hayes, C. Hill, J. Ingle, R. Jakesz, M. Kaufmann, P. McGale, L. Norton, Y. Ohashi, S. Paik, E. Perez, R. Peto, M. Piccart, L. Pierce, G. Pruneri, K. Pritchard, V. Raina, P. Ravdin, J. Robertson, E. Rutgers, Y. F. Shao, S. Swain, C. Taylor, P. Valagussa, G. Viale, T. Whelan, E. Winer, Y. Wang, W. Wood. Medical Oncology EBCTCG OVERVIEW Oxford Secretariat Richard Peto Sarah Darby Mike Clarke Christina Davies Paul McGale Richard Gray Rory Collins Jon Godwin Medical Oncology EBCTCG OVERVIEW Steering Committee - Executive Marc Buyse Mike Clarke Rory Collins Sarah Darby Christina Davies Marianne Ewertz Martine Piccart Kathy Pritchard Eric Winer William Wood Medical Oncology EBCTCG OVERVIEW Past Chairs I. Craig Henderson William Wood Current Co-Chairs Kathy Pritchard Martine Piccart Medical Oncology EBCTCG September 2010. Preliminary results Medical Oncology EBCTCG OVERVIEW 1984 First overview process data sought from all randomized trials of systemic adjuvant therapy meta-analysis concept collaboration sought built sustained Trialists Secretariat Medical Oncology EBCTCG OVERVIEW Methodology Individual patient data dates of randomization treatment allocation age menopausal status nodes ER, PgR Medical Oncology EBCTCG OVERVIEW Methodology Data checked for internal consistency Data amended and updated by correspondence Medical Oncology EBCTCG OVERVIEW Methodology Each trial analysed separately Women in one trial are compared directly with only the women in the same trial One log rank statistic per trial Stratified by age and nodal status Combined to give an overall estimate of the effect of different treatments Medical Oncology EBCTCG OVERVIEW Outcomes Recurrence first reappearance of breast cancer includes contralateral breast cancer Medical Oncology EBCTCG OVERVIEW Outcomes Deaths unknown causes included with deaths from breast cancer unless specifically stated otherwise problem of death without recurrence Medical Oncology EBCTCG OVERVIEW Outcomes Breast Cancer Related Deaths deaths of/with breast cancer Medical Oncology EBCTCG OVERVIEW Outcomes Other Deaths cardiac stroke other cancers Medical Oncology EBCTCG OVERVIEW 1984 Tamoxifen improved survival CMF chemotherapy improved survival Ovarian ablation improved survival Medical Oncology EBCTCG OVERVIEW 1990 longer tamoxifen seemed better tamoxifen effects greater in ER+ve women tamoxifen reduced rate of contralateral breast cancer chemo effective in older and younger women Medical Oncology EBCTCG OVERVIEW 1995 huge magnitude of effect of 5 years of tamoxifen 5 years of tamoxifen clearly better than 1 or 2 tamoxifen prevented contralateral breast cancer only in women with ER+ve disease anthracycline containing regimens better than CMF Medical Oncology EBCTCG OVERVIEW 2000 15 year effects of chemo sustained in older and younger women chemo effect appears greater in ER negative than in ER positive disease But is this really true? Medical Oncology EBCTCG OVERVIEW 2000 15 year effects of 5 years of tamoxifen sustained and of great magnitude door opened to question of 5 years versus longer tamoxifen ovarian suppression/ablation effective but not significantly so when added to chemotherapy Medical Oncology EBCTG OVERVIEW 2005 2000 Overview: Lancet, 2005 Trialists meet: new Steering Committee formed Many new trials added More women-years of follow-up for all major questions But major trials still missing Medical Oncology EBCTCG OVERVIEW 2006 Trialists met: new questions type of anthracycline-based regimen taxane trial status aromatase inhibitors trastuzumab chemoendocrine therapy (only in ER+, pre- and postmenopausal subsets) Subcommittees of the SC formed Medical Oncology EBCTCG OVERVIEW 2010 Tamoxifen AI’s Chemotherapy Locoregional therapy Medical Oncology 2010 EBCTCG OVERVIEW TAMOXIFEN TAMOXIFEN VS NOT No of women 1 yr vs not 2 yr vs not 5 yr vs not LONGER VS SHORTER TAM No of women 9126 23940 21457 2 – 4 vs 1 – 2 y 3200 5 vs 1 - 2 y 20000 10 vs 5 y 22000 54523 45200 Median follow-up = 15y 22% are ER- PR- Median follow-up = 5y 50% are ER ? Medical Oncology Medical Oncology 2010 EBCTCG OVERVIEW Tamoxifen for 5y vs same management but No Tam Risks Benefits (all) (ER+) Death w/o recurrence * RR 1.05 (+ 0.07) 2p>0.1 Endometrial incidence Absolute gain RR 2.33 (+ 0.25) at 15y Proportional risk reductions 2p<0.00001 • Recurrence 38% (2p<0.00001) 13% •BC mortality 30% (2p<0.00001) 9% • All deaths 22% (2p<0.00001) • Contralateral BC 39% (2p<0.00001) * Numerical excess of deaths due to stroke, pulmonary embolus, uterine cancer (15 vs 13 ; 6 vs 0; 8 vs 1) Medical Oncology 2010 EBCTCG OVERVIEW Tamoxifen for 5y vs same management but no Tam Learning more about Tam benefits On types of B.C. events… In subgroups In relation to chemotherapy administration Over time… Medical Oncology 2010 EBCTCG OVERVIEW Tamoxifen for 5y : Impact on BC events Medical Oncology 2010 EBCTCG OVERVIEW Tamoxifen for 5y: Benefits in subgroups Medical Oncology 2010 EBCTCG OVERVIEW Tamoxifen for 5y vs same management but no Tam Medical Oncology 2010 EBCTCG OVERVIEW Tamoxifen for 5y : Benefits in subgroups AGE Tumor grade Nodal status TAM for 5y : Tumor diameter BENEFITS for whom ? All do benefit !! Medical Oncology 2010 EBCTCG OVERVIEW Medical Oncology 2010 EBCTCG OVERVIEW Medical Oncology 2010 EBCTCG OVERVIEW Tamoxifen for 5y: Benefits in subgroups Yes No ER- PR- ER- PR+ Uncertain ER levels (fmol/mg prot) ER+ PR+ TAM for 5y : BENEFITS for whom ? ER+ PR- Yes Medical Oncology 2010 EBCTCG OVERVIEW Tamoxifen for 5y : Benefit over time Medical Oncology Medical Oncology 2010 EBCTCG OVERVIEW Duration of adjuvant Tam and outcome Medical Oncology 2010 EBCTCG OVERVIEW Impact of TAM duration Even 1y only provides significant benefit 10y provide small benefit which could ↑ Medical Oncology over time 2010 EBCTCG OVERVIEW Tamoxifen for 10y : Benefits vs risks at 10 y Mean follow-up only 5y Risks Benefits Absolute Xcess Absolute gain Proportional risk reductions Death w/o recurrence * • Recurrence 8% (2p=0.03) 1% (2p 0.03) + 1.5% (2p=0.59) • BC mortality 10% (2p>0.1) 2.9% (2p 0.55) Endometrial cancers • Contralateral 1.3% (2p 0.03) + 0.7% (2p=0.00004) BC *Numerical excess of deaths due to cerebrovascular events (42 vs 38 in y0-4; 27 vs 24 in y5-10), thrombo-embolic events (10 vs 56 in y0-4), end. cancers (8 vs 6 in y0-4, 4 vs 2 in y5-10) Medical Oncology 2010 EBCTCG OVERVIEW TAMOXIFEN 5y in ER+ disease reduces recurrence by 38%, BC death by 30% all deaths by 22% contralateral BC by 40% benefits all women with ER+ disease unclear benefits in ER-PgR+ disease benefits women with ER very rich tumors more increases endometrial cancer by 2.3 fold Medical Oncology 2010 EBCTCG OVERVIEW TAMOXIFEN 10 yrs vs 5 yrs of adjuvant TAMOXIFEN in ER+/? Disease absolute reduction in recurrence by 8% (2p=0.03) reduces contralateral BC by 10% (2p=0.03) increases endometrial cancer by 4 fold reduces BC mortality by 3% (2p=0.55) increases death without recurrence by 1.5% (2p=0.59) Medical Oncology 2010 EBCTCG OVERVIEW TAMOXIFEN Messages for clinical practice in 2010 PgR does not predict for benefit of adjuvant TAM For ER-PgR+ patients, the tumor should be retested and if doubt remains, TAM could be offered Medical Oncology 2010 EBCTCG OVERVIEW TAMOXIFEN Messages for clinical practice in 2010 There is presently little incentive to prescribe more than 5y of TAM, in postmenopausal women More than 5y of TAM may be useful at least for DFS in premenopausal women especially those without a uterus Medical Oncology Aromatase inhibitors EBCTCG Medical Oncology Data from 1st analysis • No unplanned cross-over • Cut-off 30 Sept 2006 • Cohort 1: 5yrs AI vs 5yrs tam • Cohort 2: 2-3 yrs of AI vs 2-3 yrs of tam after 2-3 yrs tam Medical Oncology 5 years AI vs tamoxifen: life table curve, recurrence Medical Oncology JCO, 2010, 28, 509-5 5 years AI vs tamoxifen: subgroup analysis, recurrence Medical Oncology JCO, 2010, 28, 509-5 5 years AI vs tamoxifen: life table curves, br ca mortality Medical Oncology JCO, 2010, 28, 509-5 2-3yr AI vs tam after 2-3 yrs tam: life table curve, recurrence Medical Oncology JCO, 2010, 28, 509-5 2-3yr AI vs tam after 2-3 yrs tam: subgroup analysis, recurrence Medical Oncology JCO, 2010, 28, 509-5 2-3yr AI vs tam after 2-3 yrs tam: life table curve, br ca mortality Medical Oncology JCO, 2010, 28, 509-5 2010 EBCTCG OVERVIEW Aromatase Inhibitors Message for Clinical Practice in 2010 AIs > tamoxifen recurrence survival good given early after 2 yrs Medical Oncology Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Published online December 6, 2011 in The Lancet DOI:10.1016/S0140-6736(11)61625-5 Medical Oncology EBCTCG, Lancet 2011 Direct and indirect comparisons between different polychemotherapy regimens, based on ~100,000 randomised women 45,000 taxane vs no taxane* (44,000 with anthracycline in both arms) 22,000 anthracycline vs CMF (18,000 vs “standard” CMF) 5,000 more vs less anthracycline (2000 comparing currently relevant doses) 31,000 polychemotherapy vs no adjuvant chemo (13,000 CMF vs Nil; 10,000 anthr.-based regimen vs Nil) * Excludes trials of one taxane regimen vs another Medical Oncology EBCTCG, Lancet 2011 Trials of chemotherapy vs no adjuvant chemotherapy - Any anthracycline-based regimen (eg, standard 4AC) vs nil - Standard CMF vs nil Medical Oncology EBCTCG, Lancet Chemotherapy vs no adjuvant chemotherapy L: anthracycline-based regimen (eg, standard 4AC), R: standard CMF Medical Oncology EBCTCG, Lancet Chemotherapy vs no adjuvant chemotherapy L: anthracycline-based regimen (eg, standard 4AC), R: standard CMF Medical Oncology EBCTCG, Lancet 2011 Chemotherapy vs no adjuvant chemotherapy L: anthracycline-based regimen (eg, standard 4AC), R: standard CMF Medical Oncology EBCTCG, Lancet 2011 Breast cancer mortality ratio: anthracycline-based regimen (eg, standard 4AC) or standard CMF vs no chemotherapy, by TYPE of treatment comparison Medical Oncology EBCTCG, Lancet 2011 Chemotherapy (anthracycline-based regimen or standard CMF) + 5 year endocrine therapy vs 5 year endocrine therapy only, ER+ disease only: by ENTRY AGE Medical Oncology EBCTCG, Lancet 2011 Trials of any anthracycline-based regimen (eg, standard 4AC) vs no adjuvant chemotherapy: Subgroup analyses by age, stage and ER status, and by subsets of ER+ disease Medical Oncology EBCTCG, Lancet 2011 Any anthracycline-based regimen (eg, standard 4AC) vs no adjuvant chemotherapy, by ENTRY AGE Medical Oncology EBCTCG, Lancet 2011 Any anthracycline-based regimen (eg, standard 4AC) vs no adjuvant chemotherapy, by NODAL STATUS Medical Oncology EBCTCG, Lancet 2011 Breast cancer mortality ratio: any anthracycline-based regimen (eg, standard 4AC) vs no adjuvant chemotherapy, by AGE and STAGE Medical Oncology EBCTCG, Lancet 2011 Any anthracycline-based regimen (eg, standard 4AC) vs no adjuvant chemotherapy, by ER STATUS Medical Oncology EBCTCG, Lancet 2011 Breast cancer mortality ratio: any anthracycline-based regimen (eg, standard 4AC) vs no adjuvant chemotherapy, by ER STATUS and subsets of ER+ Medical Oncology EBCTCG, Lancet 2011 Any anthracycline-based regimen (eg, standard 4AC) vs no adjuvant chemotherapy, ER+ disease only: by ENTRY AGE Medical Oncology EBCTCG, Lancet 2011 Trials of any anthracycline-based regimen* vs standard CMF *Standard 4AC, standard 4EC, or higher-cumulative-dosage regimens (eg, CAF or CEF) Medical Oncology EBCTCG, Lancet 2011 Definitions of “standard” CMF and 4AC (mg/m2 x frequency/cycle) Standard CMF: Six 4-weekly cycles of C100x14 oral M40x2 iv F600x2 iv Standard 4AC: Four 3-weekly cycles of A60 iv C600 iv Approximate equivalence: in the trials of standard AC vs standard CMF, both appeared to be of comparable efficacy Medical Oncology EBCTCG, Lancet 2011 Standard 4AC vs standard CMF: approximate equivalence Medical Oncology EBCTCG, Lancet 2011 Examples of higher-cumulative-dosage* anthracycline-based regimens (mg/m2 x frequency/cycle) CAF: Six 4-weekly cycles of C100x14 oral A40x2 iv F500x2 iv CEF: Six 4-weekly cycles of C75x14 oral E60x2 iv F500x2 iv * Higher dosage than standard 4AC not only of anthracycline but also of other cytotoxic drugs; scheduled dosages could be reduced for toxicity Medical Oncology EBCTCG, Lancet 2011 Anthracycline-based regimens with higher cumulative dosage (eg CAF/CEF) vs standard CMF Medical Oncology EBCTCG, Lancet 2011 Breast cancer mortality ratio: anthracycline-based regimen vs standard CMF, by TYPE of treatment comparison Medical Oncology EBCTCG, Lancet 2011 Trials of any anthracycline-based regimen vs standard CMF: subgroup analyses by age, stage and ER status Medical Oncology EBCTCG, Lancet 2011 Breast cancer mortality ratio: anthracycline-based regimen vs standard CMF, by AGE and STAGE Medical Oncology EBCTCG, Lancet 2011 Breast cancer mortality ratio: anthracycline-based regimen vs standard CMF, by ER STATUS and subsets of ER+ Medical Oncology EBCTCG, Lancet 2011 Taxane trials Data on 44,000 women in randomised trials of a taxane-plus-anthracycline-based regimen vs the SAME, or MORE, non-taxane chemotherapy 11,000 in trials where the non-taxane regimen was the SAME, and 33,000 in trials where it was MORE [15% node-negative; mean follow-up only 5 years; mean recurrence rate about 5% per year] Medical Oncology EBCTCG, Lancet 2011 Taxane-plus-anthracycline-based regimens vs (L) the SAME, or (R) MORE, non-taxane chemo. Medical Oncology EBCTCG, Lancet 2011 Taxane-plus-anthracycline-based regimens vs (L) the SAME, or (R) MORE, non-taxane chemo. Medical Oncology EBCTCG, Lancet 2011 Taxane-plus-anthracycline-based regimens vs (L) the SAME, or (R) MORE, non-taxane chemo. Medical Oncology EBCTCG, Lancet 2011 Taxane comparisons, subdivided according to: (a) how the non-taxane treatments compare (active = control, active = ½ control, or an intermediate ratio), and (b) whether the cycles of taxane are given concurrently (©) with the anthracycline, or whether taxanes are given alone (†). Medical Oncology EBCTCG, Lancet 2011 Breast cancer mortality ratio in taxane trials, by TYPE of treatment comparison Medical Oncology EBCTCG, Lancet 2011 Taxane trials: subgroup analyses by age, stage and ER status Taxane-plus-anthracycline-based regimen vs an anthracycline-based control regimen with the SAME, or MORE, of each non-taxane cytotoxic drug Medical Oncology EBCTCG, Lancet 2011 Taxane-plus-anthracycline-based regimen vs the SAME, or MORE, non-taxane chemo, by ENTRY AGE Medical Oncology EBCTCG, Lancet 2011 Taxane-plus-anthracycline-based regimen vs the SAME, or MORE, non-taxane chemo, by NODAL STATUS before chemotherapy Medical Oncology EBCTCG, Lancet 2011 Breast cancer mortality ratio in taxane trials, by AGE and STAGE Medical Oncology EBCTCG, Lancet 2011 Taxane-plus-anthracycline-based regimen vs the SAME, or MORE, non-taxane chemo, by ER STATUS Medical Oncology EBCTCG, Lancet 2011 Breast cancer mortality ratio in taxane trials, by ER STATUS and subsets of ER+ Medical Oncology EBCTCG, Lancet 2011 Halving big risks and halving small risks by chemotherapy • Proportional risk reduction does not depend much on age, ER status or nodal status (or on tumour grade or tumour diameter) • Absolute risk reduction, however, depends on the prognosis – and, for ER+ disease, this is the prognosis with endocrine therapy • Information lacking on tumour gene expression and on quantitative immunohistochemistry Medical Oncology EBCTCG, Lancet 2011 Effect of Radiotherapy after Breast-conserving Surgery on 10-year Recurrence and 15-year Mortality in Women with Early Breast Cancer EBCTCG September 2010. Preliminary results Medical Oncology Proportional effect of radiotherapy after breast-conserving surgery (BCS ± RT) 11 000 women, pN0/pN+/pN? Any recurrence Breast cancer mortality EBCTCG September 2010. Preliminary results Medical Oncology Absolute effect of radiotherapy after breast conserving surgery (BCS ± RT): 11 000 women pN0/pN+/pN? Any recurrence Breast cancer mortality Any death “One-in-four rule” one breast cancer death avoided for every 4 recurrences avoided EBCTCG September 2010. Preliminary results Medical Oncology Effect of radiotherapy after breast-conserving surgery (BCS ± RT): 1100 pN+ women Any recurrence Breast cancer mortality “One-in-four rule” one breast cancer death avoided for every 4 recurrences avoided EBCTCG September 2010. Preliminary results Medical Oncology Absolute effect of radiotherapy after breastconserving surgery (BCS ± RT): 7300 pN0 women Any recurrence Breast cancer mortality “One-in-four rule” one breast cancer death avoided for every 4 recurrences avoided EBCTCG September 2010. Preliminary results Medical Oncology Conclusions • Radiotherapy highly effective in reducing recurrence in both pN0 and pN+ women • Radiotherapy also reduces 15-year breast cancer • “One-in-four” rule applies for pN0 and pN1 women • Benefits not substantially reduced by fatal side-effects EBCTCG September 2010. Preliminary results Medical Oncology The Oxford Overview: Is it Still Relevant in 2010 ? Medical Oncology YES Medical Oncology EBCTCG OVERVIEW Tamoxifen 5 +/- 5 years 30% - 40% in recurrence 25 in deaths Medical Oncology EBCTCG OVERVIEW AIs Better than Tam mortality for all subgroups 25% in recurrence 0 – 25% in BC Medical Oncology EBCTCG OVERVIEW AIs ? Stronger effect after two years of tamoxifen Medical Oncology EBCTCG OVERVIEW Chemotherapy vs None CMF/AC 20 – 30% 10 – 30% recurrence BC mortality A vs CMF Medical Oncology EBCTCG OVERVIEW Adriamycin vs Standard CMF 10 – 20% recurrence 10 – 20% mortality Medical Oncology EBCTCG OVERVIEW Taxanes vs Non-Taxanes 10 – 20% 10% recurrence BC mortality Medical Oncology EBCTCG OVERVIEW Natural History of Breast Cancer ER/PgR +ve ER and PgR -ve Medical Oncology EBCTCG OVERVIEW By having all data avoids publication bias gives average effect size clarifies time frames of effects process / outcomes both useful Medical Oncology EBCTCG OVERVIEW Future – Yes Publications 2 on radiation results 2010 - 2011 one on chemotherapy 2011 one on tamoxifen 2011 one on AIs 2011 Meet Again September 19-22, 2012 Medical Oncology Medical Oncology Medical Oncology Medical Oncology Medical Oncology EBCTCG OVERVIEW Future – Yes Publications 2 on radiation results 2010 - 2011 one on chemotherapy 2011 one on tamoxifen 2011 one on AIs 2011 Meet Again September 19-22, 2012 Medical Oncology EBCTCG September 2010. Preliminary results Medical Oncology